 |

School of Biomedical Sciences, Faculty of Medicine and Health Sciences, Queens Medical Centre, Nottingham NG7 2UH, UK. |
 |
 |

School of Biomedical Sciences, Faculty of Medicine and Health Sciences, Queens Medical Centre, Nottingham NG7 2UH, UK. |
 |
Peptidyl-Prolyl Isomerases
|
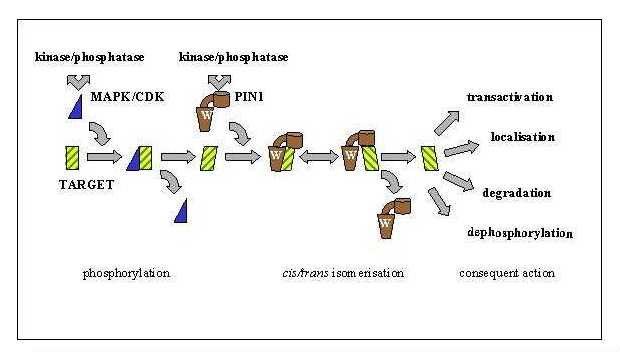
Peptidyl-Prolyl Isomerases (PPIs) catalyse the cis-trans isomerisation of peptide bonds amino-terminal to proline residues in polypeptide chains. The cis and trans forms of peptidyl-proline bonds exist in equilibrium, whereby it is estimated from protein structure databases that approximately 7% of peptidyl proline bonds exist in the cis conformation. One group of eukaryotic PPIs, the parvulin-like family, includes members that are involved in cell cycle control. For example deletion of Ess1 in Saccharomyces cerevisiae causes cell cycle arrest in G2 and is effectively lethal. The human homologue Pin1 is able to rescue these phenotype. |
|
As well as a catalytic domain, both Ess1 and Pin1 proteins contain a WW domain that has been shown to bind selectively to phosphorylated motifs (either pSer-Pro or pThr-Pro) in their target proteins. This means that Ess1/Pin1 activity is regulated by prior phosphorylation. The pSer-Pro or pThr-Pro motifs recognise by Ess1/Pin1 are generated by the action of Cyclin Dependent Kinases (CDKs) or Mitogen-Activated Protein Kinases (MAPKs) in response to their activation during the cell cycle. |
 |
Reference
Shaw, P.E. (2002) Peptidyl-Prolyl Isomerases: A New Twist to Transcription. EMBO Reports 3, 521-526