 |
Neuronal Networks
|
 |
 |
Neuronal Networks
|
 |
More recently, the advent of integrated-circuit fabrication technologies, including microetching and metal deposition, has resulted in the production of multi-electrode devices on planar substrates.
The essential requirements for a working multiple-microelectrode to record from biological tissue include:
Gross and colleagues (1977) were among the first to demonstrate that single-unit neuronal activity could be recorded in vitro with a photoetched 36-microelectrode array. The array consisted of tracks on a glass substrate, which initially were entirely covered by a thin insulating layer. The tracks were then deinsulated at their ends by a pulsed laser beam, exposing electrode contacts 12 m m in diameter. This array (referred to by Gross as a multiple-microelectrode plate (MMEP) or more recently as a multiple-microelectrode array (MMEA) allowed the simultaneous recording of activity from several units in an excised snail brain ganglion (Gross, 1979) and later mouse spinal cord (Gross and Lucas, 1982). A major advantage of this system is that it also permitted optical monitoring of neuronal culture growth, thus allowing the growth to be correlated with the appearance of electrical activity (Gross and Lucas, 1982). Pine and Gilbert (1982) independently developed a similar device. Their MMEA substrate was a rectangular glass wafer with dimensions 40.4 mm x 24 mm x 0.44 mm. The array of 61 electrodes, each 10 mm in diameter and spaced 70 m m apart, was centrally positioned on the glass substrate in a hexagonal conformation. Conducting tracks, made of indium-tin oxide, ran from the electrodes to the long edges of the wafer, were contact was made to the pre-amplifiers. The entire surface of the wafer, except the electrodes and the edge contacts, were insulated with a thin polyimide layer. As with the Thomas et al. MMEA electrode, impedance was reduced by coating the electrodes with platinum black, resulting in a typical impedance of 100 kW at 1 kHz.
More recently there has been a massive increase in the number of different multiple microelectrode devices available for in vitro recording (e.g., Novak and Wheeler, 1988; Jimbo and Kawana, 1992; Nisch et al., 1994; Stoppini et al, 1997). Many multi-microelectrode devices also exist which permit in vivo recordings (e.g. González and Rodríguez, 1997) however these devices will not be reviewed here - further information may be found at: Center for Neural Communication Technology (University of Michigan).
[1] The microelectrode arrays (MMEPs) currently used in our laboratory for studies of random self-organising networks, derived from dissociated hippocampal neurones, are an evolution of those first developed and supplied by Guenter Gross at the Center for Network Neuroscience (University of North Texas, USA). The electrodes plates used are known as Multi-MicroElectrode Plates (Fig. 1). The construction of these devices is fully described by Gross and Kowalski (1991) and Gross et al. (1985) and briefly described below - contact Guenter at Center for Network Neuroscience (University of North Texas) for further information.

Figure 1: Configuration of a 64-channel MMEP microelectrode array (x70)
Glass squares measuring 50 mm x 50 mm x 1.1 mm were coated with thin film indium-tin oxide (ITO) coatings using DC sputtering techniques. Glass sheets were chosen over fragile cover slips to minimise breakage and optical distortion when mounted in the recording chamber. A pattern of 64 conductors terminating in 4 rows of 16 electrodes in a centrally positioned 1.2 mm2 recording area (0.6 mm x 0.6 mm) was achieved using standard positive photoresist photolithographic techniques. The conductors were 8 m m wide in the recording area and gradually widened until they reached 32 contact pads at either side of the plate where contact was made with the amplifier circuit boards via carbon elastomere "zebra strips" connectors (Fujipoly, New Jersey, USA).
The MMEPs were then insulated in two steps with a polysiloxane resin: firstly a 3 m m layer was spun on at 4400 rpm and a thicker, second insulating layer (50-60mm) was then hand-painted on either side of the recording matrix to within 1 mm from the edge of the central recording area. The second layer helped to raise the shunt impedance of the individual conductors from 5 MW to 25 MW . New-generation MMEPs (supplied late 1997 onwards) only posses a single 1.5-2 mm thick insulating layer. The MMEP was then laser-deinsulated in the recording area with single 12 ns pulses (wavelength=337 nm) from a pulsed UV laser. This exposed an ITO surface measuring approximately 8 x 10 m m. Electrode impedance was then lowered to 2-4 MW by applying colloidal gold to the individual microelectrodes (Gross et al., 1985).
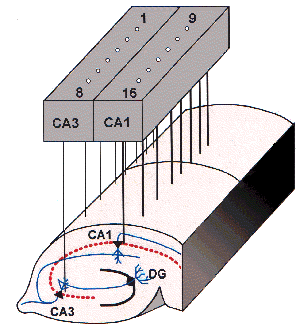
[2] The microelectrode arrays (MEAs)
currently used in our laboratory for studies of networks in organotypic hippocampal
cultures comprises the 60-channnel arrays supplied by MultiChannel Systems (Germany)
- further information on these devices (e.g. Nisch et al (1994); Egert et al
(1998)) may be found at: Multi-Channel
Systems.
[3] Some in-house MMEAs are being developed and tested in collaboration with colleagues in the School of Physics and Division of Material Engineering & Material Design at Nottingham.
[4] Micro-wire arrays (see chapters 1 and 2 Nicolelis et al, 1999)
Egert, U., Schlosshauer, B., Fennrich, S.,
Nisch, W., Fejtl, M., Knott, T., Muller, T. and Hammerle, H. (1998) A novel
organotypic long-term culture of the rat
hippocampus on substrate-integrated multielectrode arrays.
Brain Research Protocols 2: 229-242.
González C and Rodríguez M. (1997) A flexible perforated microelectrode array probe for action potential recording in nerve and muscle tissues. J. Neurosci. Methods. 72, 189-195.
Gross GW, Rieske E, Kreutzberg GW and Meyer A. (1977) A new fixed-array multi-microelectrode system designed for long-term monitoring of extracellular single unit neuronal activity in vitro. Neurosci. Lett. 6, 101-106.
Gross GW. (1979) Simultaneous single unit recording in vitro with a photoetched laser deinsulated gold multimicroelectrode surface. IEEE TransBiomed. Eng. 26, 273-278
Gross GW and Lucas JH. (1982) Long-term monitoring of spontaneous single unit activity from neuronal monolayer networks cultured on photoetched multielectrode surfaces. J. Electrophys. Tech. 9, 55-69.
Gross GW and Kowalski JM. (1991) Experimental and theoretical analysis of random nerve cell dynamics. In: Neural Networks: Concepts, Applications, and Implementations. Eds: Antognetti P, and Milutinovic V. Prentice Hall, New Jersey. pp 47-110.
Gross GW, Wen W and Lin J. (1985) Transparent indium-tin oxide patterns for extracellular, multisite recording in neuronal culture. J. Neurosci. Methods15, 243-252.
Hanna GR and Johnson RN. (1968) A rapid and simple method for the fabrication of arrays of recording electrodes. Electroencephalogr. Clin. Neurophysiol.25, 284-286.
Jimbo Y and Kawana A. (1992) Electrical stimulation and recording from cultured neurones using a planar electrode array. Bioelectrochem and Bioenergetics29, 193-204.
Jobling DT, Smith JB and Wheal HV. (1981) Active microelectrode array to record from the mammalian central nervous system in vitro. Med. Biol. Eng. Comput. 19, 553-560.
Nicolelis MAL, Stambaugh CR, Brisben A and Laubach M (1998) In Methods for neural ensemble recordings, Eds. Nicolelis MAL, CRC Press, p121-156.
Nisch W, Böck J, Egert U, Hämmerle H and Mohr A. (1994) A thin film microelectrode array for monitoring extracellular neuronal activity in vitro. Biosensors and Bioelectronics. 9, 737-741.
Novak JL and Wheeler BC. (1988) Multisite hippocampal slice recording and stimulation using a 32 element microelectrode array. J. Neurosci. Methods 23, 149-159.
Pine J and Gilbert J. (1982) Studies of cultured cells in dishes incorporating integral microcircuit electrodes. Soc. Neurosci. Abst. 8, 670.
Prohaska O, Olcaytug F, Womastek K and Petsche H. (1977) A multielectrode for intra-cortical recordings produced by thin-film technology. Electroencephalogr. Clin. Neurophysiol. 42, 421-422.
Scharfman HE. (1994) Synchronisation of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neurosci. 59, 245-257.
Stoppini L, Duport S and Corrèges P. (1997) A new extracellular multirecording system for electrophysiological studies: application to hippocampal organotypic cultures. J. Neurosci. Methods. 72, 23-33.
Thomas CA Jr, Springer PA, Loeb GE, Berwald-Netter Y and Okun CM. (1972) A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell. Res. 74, 61-66.
Verzeano M. (1956) Activity of cerebral neurons in the transition from wakefulness to sleep. Science124, 366-367.
Rob Mason/David Sokal/Mandy
Edwards
Last Revised: 20-Jul-2000