Project summary
A Brief History of Somatotopy
Wilder Penfield1 created highly accurate maps of S1 (somatosensory cortex) by electrically stimulating the brains of human participants, invasively, via 'osteoplastic craniotomy', in 1937. This, and other studies2expanding Penfield's findings, revealed a somatotopic representation of S1, much like the retinotopic nature of V1.
Retinotopy is typically performed by presenting expanding checkerboard and rotating wedge stimuli to a participant during an fMRI experiment3. Eccentricity and Polar angle can be accurately derived from the phase of the voxel's response in the ROI (in this case, visual cortex). This simple procedure can reveal the mean receptive field centre of a voxel which is activated by the stimulus.
Given the somatotopic nature of S1, different methods have been utilised to expose the underlying somatotopic activation of human and animal sensory space, with specific focus on the hands and digits. The use of air-puffs4,5, brush strokes and manual stroking6,7, whilst the participant is in the scanner have mapped S1 (specifically Brodmann areas 1, 2 and 3) to high detail. The group at Nottingham8-11 have used a piezo-electric vibratory non-magnetic device to deliver the stimulation to individual fingertips, and the phalanges of the digits. Combining this with 7T fMRI, and using a 'travelling wave' paradigm produces some of the most accurate maps in non-invasive somatotopy to date.
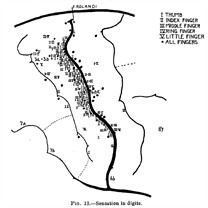
Fig1. Penfield's map of S1
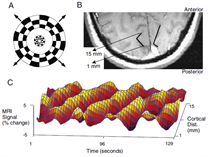
Fig2. Retinotopy performed by Engel et al. in 1994
Spatial and Temporal Mapping of Mechanosensation
- We will extend previous studies of digit somatotopy by investigating ipsilateral response to vibratory stimuli.
- We are currently adapting a visual paradigm developed in the visual field known as population receptive field mapping (pRF)12, in order to characterise receptive field sizes accurately, rather than just telling if somewhere is active or not. The pRF allows modelling of the entire voxel's response (rather than just the mean centre).
- We will also collect resting state fMRI in order to investigate correlated spontaneous signals between inter-hemispheric S1 and S2 networks, and to separate out fine-scale functional networks within sub-areas.
- As stated, these data will be acquired using 7T fMRI, but also implementing a novel technique to speed up acquisition known as simultaneous multi-slice13,14 imaging, or 'multiband'.
- Layer-specific fMRI can distinguish the laminar segregation of functionality in the brain, thus, we want to be able to test the hypothesis that ipsilateral input decreases S1 response to contralateral stimulation in layer V. Bilateral stimulation produces a reduced response in S1, relative to unilateral stimulation in cats15.
- As well as using BOLD imaging, we want to investigate arterial cerebral blood volume (aCBV) using a new technique called VASO (vascular space occupancy)16
- MEG can elucidate transient evoked responses to digit stimulation, dynamic networks of mechanosensation and can identify neural oscillations, due to its high temporal resolution. Electrophysiological studies have identified the layer-specificity of neural oscillations, i.e. gamma and alpha band activity are associated with superfical and deep cortical layers, respectively17,18. By combining layer-specific results from fMRI, we hope to combine the MEG spectral response and determine whether a direct relationship exists between the layer-specificity of MEG and laminar structure revealed by fMRI.
Intraneural microstimulation
Microneurography19 is an invasive technique that records electrical activity from single tactile afferents using electrodes inserted into a nerve. These afferents can be stimulated via interneural microstimulation (INMS)20,21, producing a distinct percept in S1 in the absence of touch.
- We will develop an INMS system for use in fMRI and MEG, allowing us to record the natural spike discharge pattern and play these back during scanning in order to characterise the perceptual impact of a naturally generated percept.
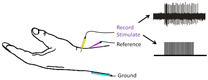
Fig3. A graphic depiction of INMS
Spatial and Temporal Re-mapping of Mechanosensation in CTS and FHD
Accurate maps and cortical signatures of healthy participants derived non-invasively by fMRI and MEG, and invasively by INMS, using using the above experiments will lead our clinical investigation. CTS (carpal tunnel syndrome) and FHD (focal hand dystonia) are the two patient populations we will be examining22-25.
- We will perform pRF mapping, determine functional connectivity (fMRI) and assess spatial and spectro-temporal dynamics of mechanosensation (MEG).
- Layer specific activity and interhemispheric communication will be investigated in patients, based on a hypothesis of abnormal plasticity.
- Finally, we will see whether longitudinal intervention therapies alter patients' somatosensory cortices (nerve decompression for CTS and botulinium toxin for FHD).
References
- Penfield, W. & Boldrey, E. Somatic Motor and Sensory Representation in the cerebral cortex of man as studied by electrical stimulation. Brain A J. Neurol. 60, 389–443 (1937).
- Woolsey, C. N. Cortical Representation of Tactile sensibility as indicated by cortical potentials. Science (80-. ). 85, 388–390 (1937).
- Engel, S. A. et al. fMRI of Human Visual Cortex. Nature 369, (1994).
- Servos, P., Zacks, J., Rumelhart, D. E. & Glover, G. H. Somatotopy of the human arm using fMRI. Neuroreport 9, 605–9 (1998).
- Stringer, E. A., Chen, L. M., Friedman, R. M., Gatenby, C. & Gore, J. C. Differentiation of somatosensory cortices by high-resolution fMRI at 7T. Neuroimage 54, 1012–1020 (2011).
- van Westen, D. et al. Fingersomatotopy in area 3b: an fMRI-study. BMC Neurosci. 5, 28 (2004).
- Martuzzi, R., van der Zwaag, W., Farthouat, J., Gruetter, R. & Blanke, O. Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus. Hum. Brain Mapp. 35, 213–26 (2014).
- Sanchez-Panchuelo, R. M., Francis, S., Bowtell, R. & Schluppeck, D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J. Neurophysiol. 103, 2544–2556 (2010).
- Sanchez-Panchuelo, R. M. et al. Within-Digit Functional Parcellation of Brodmann Areas of the Human Primary Somatosensory Cortex Using Functional Magnetic Resonance Imaging at 7 Tesla. J. Neurosci. 32, 15815–15822 (2012).
- Sánchez-Panchuelo, R.-M. et al. Regional structural differences across functionally parcellated Brodmann areas of human primary somatosensory cortex. Neuroimage 93, 221–230 (2014).
- Besle, J., Sánchez-Panchuelo, R.-M., Bowtell, R., Francis, S. & Schluppeck, D. Single-subject fMRI mapping at 7 T of the representation of fingertips in S1: a comparison of event-related and phase-encoding designs. J. Neurophysiol. 109, 2293–305 (2013).
- Dumoulin, S. O. & Wandell, B. A. Population receptive field estimates in human visual cortex. Neuroimage 39, 647–660 (2008).
- Moeller, S. et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn. Reson. Med. 63, 1144–1153 (2010).
- Feinberg, D. A. & Setsompop, K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 229, 90–100 (2013).
- Tommerdahl, M., Simons, S., Chiu, J., Favorov, O. & Whitsel, B. Response of S1 cortex to ipsilateral, contralateral and bilateral flutter stimulation in the cat. BMC Neurosci. 6, 1–10 (2005).
- Huber, L. et al. Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7T. Neuroimage 97, 349–362 (2014).
- Spaak, E., Bonnefond, M., Maier, A., Leopold, D. A. & Jensen, O. Layer-Specific Entrainment of Gamma-Band Neural Activity by the Alpha Rhythm in Monkey Visual Cortex. Curr. Biol. 22, 2313–2318 (2012).
- Maier, A., Adams, G. K., Aura, C. & Leopold, D. a. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front. Syst. Neurosci. 4, 1–11 (2010).
- Johansson, R. S., Westling, G. & Vallbo, Å. B. Thresholds of mechanosensitive afferents in the human hand as measured with von frey hairs. Brain Res. 184, 343–351 (1980).
- Löken, L. S., Wessberg, J., Morrison, I., McGlone, F. & Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 12, 547–548 (2009).
- Kelly, E. F., Trulsson, M. & Folger, S. E. Periodic microstimulation of single mechanoreceptive afferents produces frequency-following responses in human EEG. J. Neurophysiol. 77, 137–144 (1997).
- Butterworth, S. et al. Abnormal cortical sensory activation in dystonia: An fMRI study. Mov. Disord. 18, 673–682 (2003).
- Dhond, R. P. et al. Spatio-temporal mapping cortical neuroplasticity in carpal tunnel syndrome. Brain 135, 3062–3073 (2012).
- Napadow, V. et al. Somatosensory cortical plasticity in carpal tunnel syndrome-a cross-sectional fMRI evaluation. Neuroimage 31, 520–530 (2006).
- Maeda, Y. et al. Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain 137, 1741–1752 (2014).