
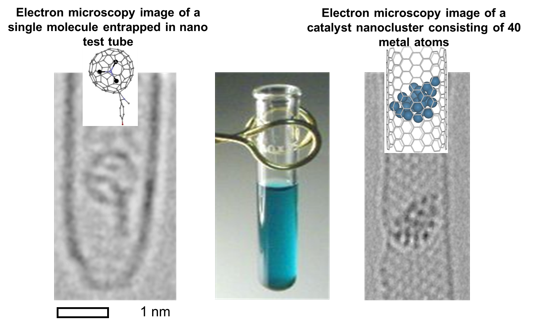
The key aim of the Nottingham Nanocarbon Group is to bridge the molecular world and the macroscopic world using nanotubes and other low-dimensional materials as a physical bridge. Atomically thin, yet mechanically robust and chemically stable carbon nanostructures, such as nanotubes, nanofibres, and graphene, have been successfully developed in our group as nanoscale test tubes (Guinness Book record for World's tiniest test tube), nanoreactors and nanoelectrodes. |
 |
 |
|
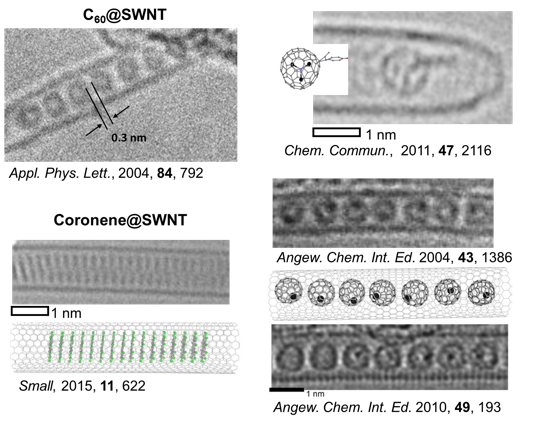
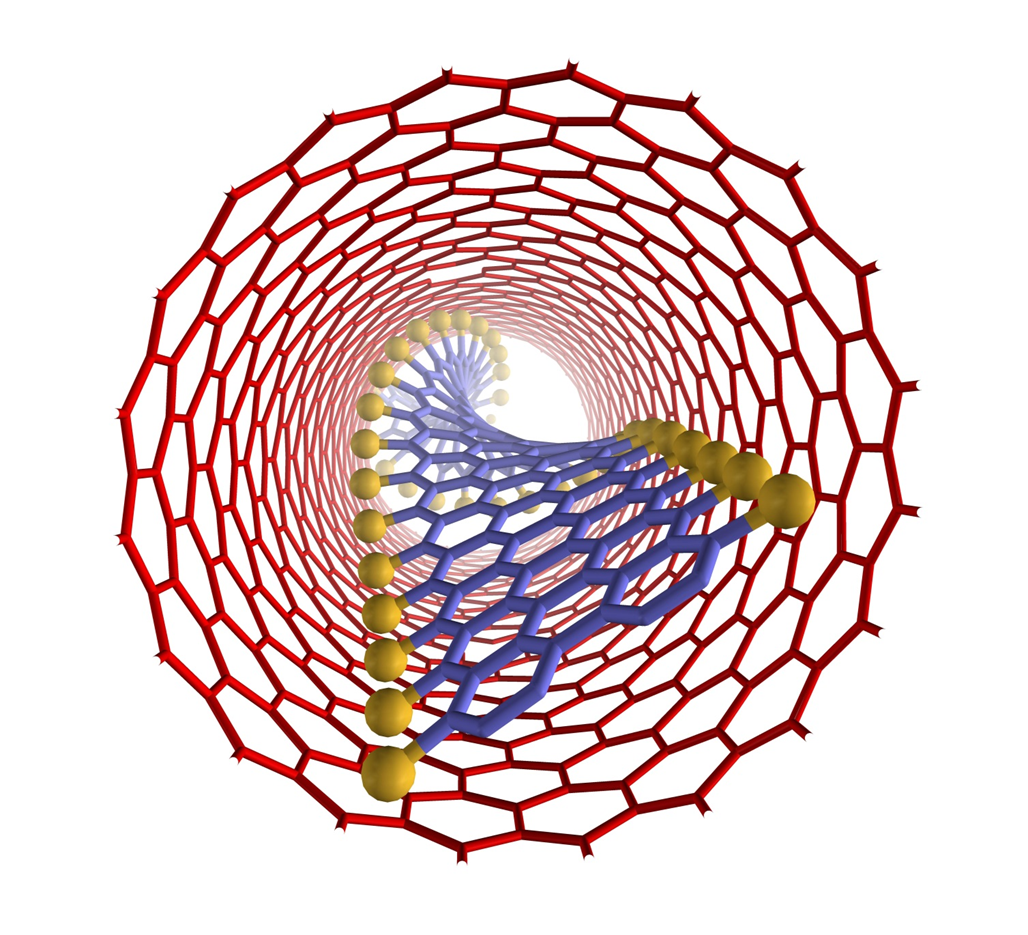
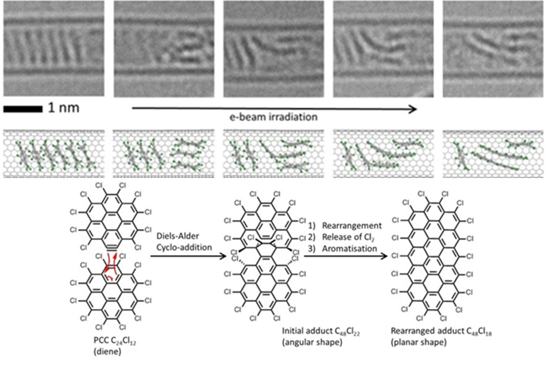
Confinements of molecules in nanotubes allows investigation of structure and reactivity of individual molecules by methods of electron microscopy and different spectroscopy techniques developed in our group, unravelling pathways of interactions and reactions at the single-molecule level. |
 |
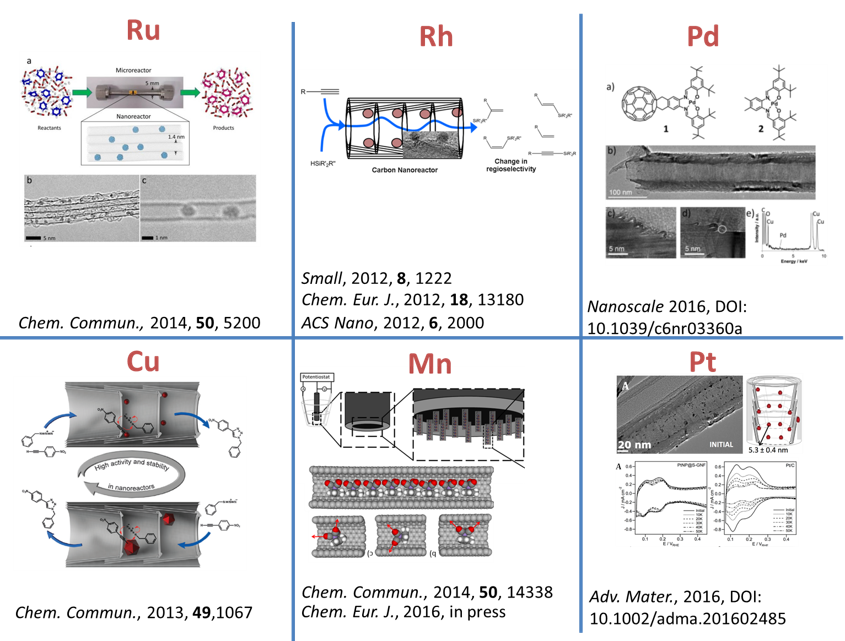 |
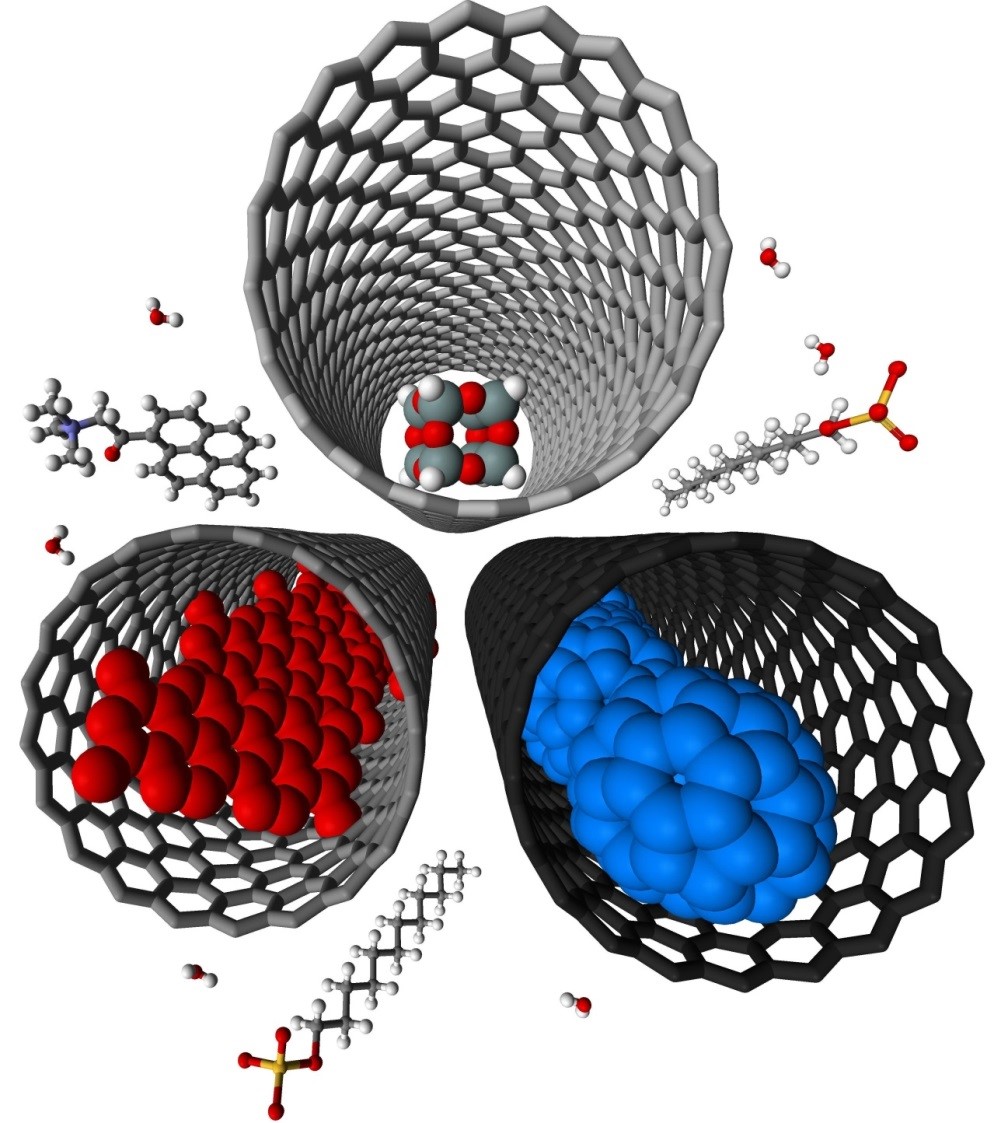
We have demonstrated potential of nano test tubes to control the positions and orientations of guest-molecules, and to tune catalytic, magnetic, electric and optical properties. In addition, synergistic interactions between molecules and carbon nanostructures offer conceptually new strategies for the regio-, chemo- and enantioselectivity control in preparative reactions. Confinement of catalysts in nanotubes extends their lifetime under harsh reaction conditions and enables effective reuse of the catalyst. In electrochemical reactions the nanotube acts not only as a nanoreactor but also as a highly effective nanoelectrode supplying electrons to the reactant molecules. |
 |
 |
|
Materials we work with: carbon nanotubes, carbon nanofibers, fullerenes, few-layer graphene, boron nitride nanotubes, metal nanoparticles, metal oxide nanoparticles, coordination and organometallic compounds. |
|
Reactions we study in nanotubes: a wide range of organic reactions (hydrosilylation, alkene oxidation, azide cycloaddition, arene halogenation, arene cross-coupling, alkene hydrogenation, electrocatalytic alcohol oxidation) and inorganic reactions (metal oxide, sulphide and iodide formation, electrocatalytic oxygen reduction). |
|
Methods we use: all standard methods of synthetic chemistry and chemical analysis (e.g. NMR, mass spec, chromatography, IR, UV-vis, powder and single-crystal XRD) augmented by electron microscopy, electrochemistry and Raman spectroscopy methods developed in our group specifically for the nanoreactors. |
|
For latest research results, please check our Publications and Popular press pages. |
|