Supercritical carbon dioxide offers many advantages for performing oxidation reactions. O2 is fully miscible with scCO2. This allows oxidations to be performed without the mass transfer limitations of traditional biphasic reactions. CO2 is also fully oxidised and it provides a safe medium for performing oxidation reactions within. Photophysical investigations by Worrall et al. (see paper) have demonstrated that it is possible to generate 1O2 in scCO2. They also showed that 1O2 has a relatively long lifetime in scCO2 (5.1 ms at 14.7 MPa and 314 K) and this lifetime was found to be dependant upon the density of the scCO2. To achieve comparable lifetimes in traditional solvents, it is necessary to use highly chlorinated solvents such as chloroform and carbon tetrachloride which are environmentally unacceptable for large scale use.
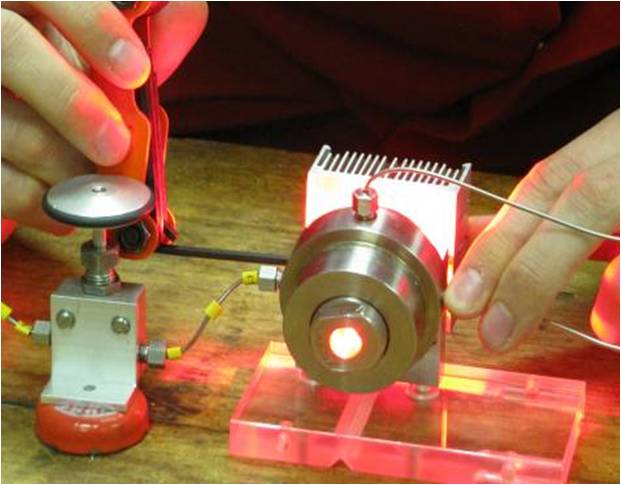
Recently through a joint effort between the George and Poliakoff groups has resulted in the first study of reactions of 1O2 with organic substrates in scCO2. Initial work was performed on the photo-oxidation of alpha-terpinene to ascaridole using a small batch scale apparatus (~1 mL). This work showed that ascaridole was formed with zero-order kinetics with a high TOF by using a highly fluorinated porphyrin molecule (TPFPP). "Homogeneous photochemical oxidation via singlet O2 in supercritical CO2" (R. A. Bourne, X. Han, A. O. Chapman, N. J. Arrowsmith, H. Kawanami, M. Poliakoff and M. W. George Chem. Commun. (2008), 4457 - 4459).
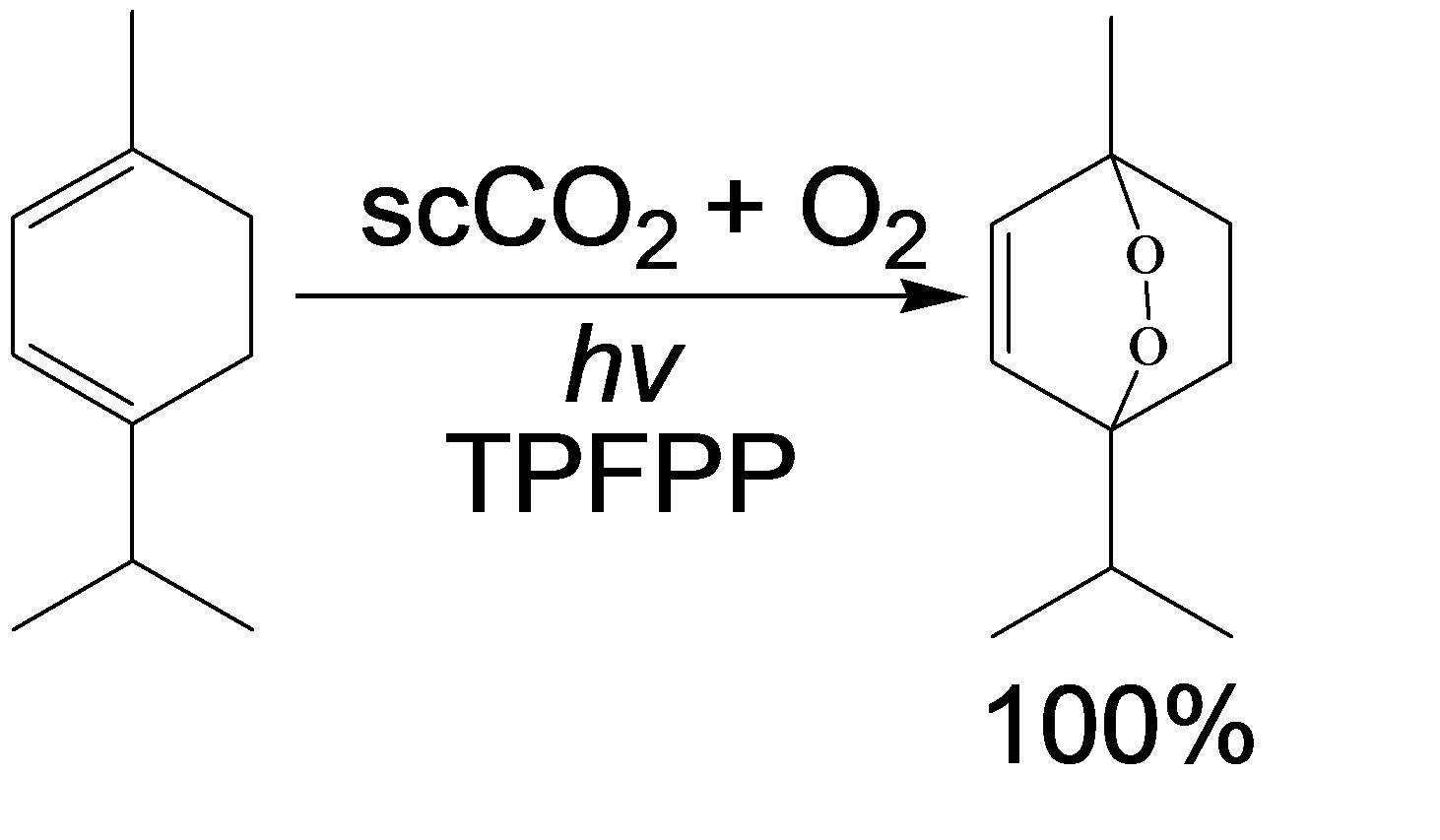
Following this initial work, we then evaluated the effectiveness of light emitting diodes (LEDs) as point light sources, for this reaction. Combining several LEDs into an array and using a specially designed sapphire reactor tube enabled us to construct an effective continuous flow reactor for performing photo-oxidation (see video for schematic below). This sapphire tube photo-reactor exhibited enhanced space time yields compared to more conventional reactors. "Cleaner Continuous Photo-Oxidation Using Singlet Oxygen in Supercritical Carbon Dioxide" (R. A. Bourne. X. Han, M. Poliakoff and M. W. George), Ang. Chem. -Int Ed., (2009), 5322-5325
Video made by www.deuterateddesigns.com.
We have most recently been using surfactants and co-solvents with a wide range of photosensitisers such as methylene blue that can be used in scCO2. "Strategies for Cleaner Oxidations Using Photochemically Generated Singlet Oxygen in Supercritical Carbon Dioxide" (X. Han, R. Bourne, M. Poliakoff and M. W. George), Green Chemistry, 2009, (11), 1787 - 1792.
We always welcome e- mails to martyn.poliakoff@nottingham.ac.uk or mike.george@nottingham.ac.uk from those interested in this area or who would like reprints of papers.
