Contact
Biography
BA University of Cambridge (Jesus) 1997, DPhil King's College, University of London 2002, Lecturer, Institute of Genetics 2002 - Present.
Research Summary
My lab is interested in the process of development and, ultimately, how cells are programmed to different phenotypes. We investigate these processes using a variety of approaches, including in vivo,… read more
myGRN - my Gene Regulatory Network

We have developed an online database to store gene regulatory interactions, compile these into networks and dynamically draw networks in real time. Our database also facilitates network topology analysis, including searching for motifs (mfinder and our own custom algorithms) and curation of data from existing sources utilising chilibot.
Our database focuses on developmental regulatory networks and we have developed several custom views for facilitating the analysis of these networks. For example, genes can be automatically positioned according to the time and location in which they are expressed (see the image to the left). Alternatively, networks can be viewed based solely on the topology of the connections between individual genes (see image to the right).
Please contact us if you are interested in generating your own networks, making large interaction datasets available through our database, or working with us to further develop these tools.
Over the coming weeks, our previous networks will be transferred across into myGRN and made publicly available. For more information on myGRN visit www.myGRN.org
For reference, links to our previous network sites are maintained below.
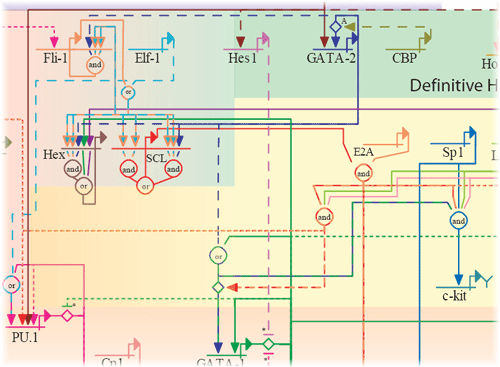
A section of the erythroid lineage network.
Genetic Regulatory Networks:
It is clear that events in the development of an organism occur in an ordered, organised and reproducible fashion. Unnderlying each step of external development is the structured expression of transcription factors, signals and targets. I am interested in uncovering and understanding these processes. How do complex networks of genes function together to regulate development?
We are currently working on three main areas, mesendoderm, hematopoiesis and lens development. In addition, we have generated networks for heart development. To explore further, please follow the links below.
The Mesendoderm Network Free Access
The mesendoderm network accompanies:
M. Loose and R. Patient (2004) A Genetic Regulatory Network for Xenopus Mesendoderm Formation. Developmental Biology 271, 469-480
Erythroid Lineage Programming and Development Free Access
The erythroid networks accompany:
G. Swiers, R. Patient and M. Loose (2006) Genetic regulatory networks programming hematopoietic stem cells and erythoid lineage specification. Developmental Biology, in press
The Lens Network (currently unavailable) (in collaboration with Seb Shimeld)
The Heart Network Free Access
The Heart Network accompanies:
T. Peterkin, A. Gibson, M. Loose and R. Patient (2005) The Roles of GATA 4, -5, and -6 in Vertebrate Heart Development. Seminars in Cell & Developmental Biology 16 (2005) 83-94
Many of the networks illustrated here were generated in collaboration with Prof Roger Patient at the MRC Molecular Hematology Unit, Oxford University.
Work in this laboratory is supported b
Current Research
My lab is interested in the process of development and, ultimately, how cells are programmed to different phenotypes. We investigate these processes using a variety of approaches, including in vivo, in vitro and in silico methods.
For information on compiling gene regulatory networks and to find out more about the tools we have developed, visit the 'Genetic Regulatory Network' pages or visit www.myGRN.org . An excellent and comprehensive review has ercently been published by Thomas Schlitt and Alvis Brazma - details available here.
Mesendoderm specification in amphibians.
The process of germ layer specification in Xenopus laevis has been extensively studied and a detailed picture describing the underlying genetic regulatory events is emerging. In part, this is a result of our work to deduce a GRN describing the specification of mesoderm and endoderm during early development ( Loose and Patient, 2004). This network, although incomplete as not all interactions have yet been experimentally verified, already appears highly complex (see below).
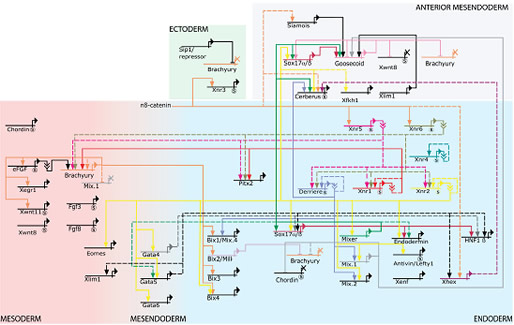
However, much of this complexity emerges from the existence of multiple copies of key developmental regulators, such as the genes of the Nodal and Mix families. We therefore reasoned this complexity results from sub-functionalisation.
One of the goals of systems biology is to make predictions about the behaviour of complex systems. In this case, the presence of multiple copies of the Nodal and Mix families makes such predictions complex. As a first step towards a predictive model of network function, we developed mathematical models describing a simplified version of this network. We simplified the network on the basis of the existence of only single copies of the Nodal and Mix gene in mouse and Human. In collaboration with John King, we developed a mathematical model of this network and demonstrated the underlying topology of the network is sufficient to generate both mesoderm and endoderm in response to changes in level of key upstream molecules. In this case, we mark mesoderm as Brachyury expressing and endoderm as Mix expressing cell types.
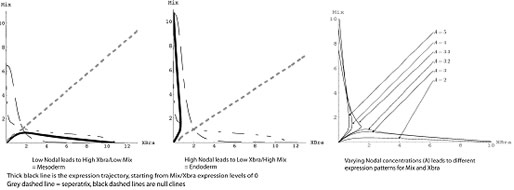
We would like to experimentally test predictions of this model, but our model is based on single genes, whilst Xenopus has multiple copies. Given that the mouse and human also have single copies of these genes, we asked if an alternative amphibian model existed with the experimental benefits of Xenopus but the simplicity of fewer genes.
A collaborator within the Institute of Genetics, Andrew Johnson, proposed that the axolotl, a urodele amphibian, is developmentally constrained by the requirement to induce germ cells. This constraint is relieved by pre-determined germ cells, where germ cell determinants (germ plasm) are deposited in the egg and distributed only to those cells that give rise to germ cells. Xenopus,