Andy Green - Glutaminase is a key therapeutic target in luminal breast cancer 2019 Pilot Grant
Lay summary
Background: Breast cancer affects over 55,000 women every year. Most patients have a type of breast cancer which responds well to present drugs and therefore live longer. However, about a quarter of these patients develop an aggressive form of breast cancer and can die earlier. This form of breast cancer doesn’t respond well to drugs and in some patients they only work for a while. In our current work, we have found a protein which is in the aggressive type of breast cancer and when it is present, patients do not respond well to drugs and patients die earlier. This protein has a key role within the cancer cell as it provides nutrients for the cancer cells to grow faster. Some cancer cells rely on this nutrient and without it the cancer cell won’t be able to grow as quickly. A drug has been developed which stops this protein working but it has not been tested in this aggressive form of breast cancer. We think that if we can target this proteins we can stop the nutrient being used by the cancer cells and stop them growing.
Aims: In this project, we aim to confirm whether we can stop this protein working in the aggressive type of breast cancer to stop them growing.
Techniques and Methodology: We will use the drug to stop the protein working in breast cancer cells and measure to see whether it stops them growing.
Impact on breast cancer research: We think that this protein can act as target for this drug which will stop the growth of these aggressive breast cancers. This will give doctors a better choice of drugs which they can give to the patients that will most likely benefit
Scientific summary
Background: There are limited effective treatments for pa0ents with aggressive oestrogen receptor positive (ER+) luminal B (lumB) breast cancer (BC) which accounts for a quarter of all BC and have a 20% risk of recurrence. The exploitation of “metabolic transformation”, a hallmark of cancer, for anticancer therapy has recently attracted great attention. Glutamine is the second most utilised amino acid aher glucose for driving tumour cell proliferation and cell survival, with some cancer cells known to exhibit glutamine addiction. A selective inhibitor to glutaminase, CB-839, is currently in clinical trials for triple negative BC. We have been the first to show that glutamine metabolism also plays an important role in the aggressive subclass of luminal BC. We therefore hypothesise that lumB BC, which can relapse or develop resistance to current treatments, will also be sensitive to glutaminase inhibition.
Aims: We aim to assess the value of glutaminase as a therapeutic target for lumB BC to confirm our hypothesis that they will be sensitive to the inhibition of glutaminase.
Techniques and Methodology: We will use the selective inhibitor, CB-839, to inhibit glutaminase and also knockdown glutaminase gene expression in a panel of lumB cell lines and measure the effects on cell growth and progression together with glutamine metabolism.
Impact on breast cancer research: This pilot study will confirm, using in vitro methods, that the selective glutaminase inhibitor, CB-839, as a potential therapeutic intervention for highly aggressive luminal BC. Ultimately it will offer a potential new therapeutic option for these patients, whom have an uncertain prognosis due to relapse and/or development of resistance to current therapies.
Research Update - 10th February 2025
What specific achievements were made possible through your pilot grant award? With the pilot grant from NBCRC, we conducted important lab tests. These tests showed that blocking two forms of a protein called glutaminase (which helps cancer cells use the nutrient glutamine) can slow down the growth of a fast-growing type of breast cancer that's called ER+.
We've already presented these exciting findings at an international conference.
How has NBCRC membership benefitted your research and career? My membership in NBCRC has allowed me to work closely with a large network of experts – scientists and clinicians – who are passionate about advancing breast cancer care
Could you provide a brief update on your current research progress? Now, we're writing up all the details in a research paper so other scientists can learn from our work. We're hoping to secure more funding so we can continue this exciting research and make even more progress.
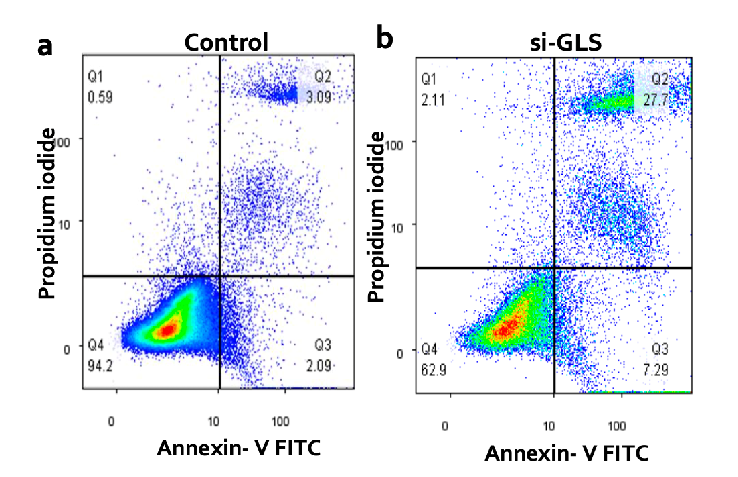
We studied how lowering the level of the GLS protein affectscancer cell death (called apoptosis) in breast cancer cells. We used a technique called flow cytometry to measure cell death in cells where we reduced GLS (called "knockdown" cells) and in control cells (where GLS levels were normal). The pictures a and b show examples of the results from these flow cytometry tests comparing the control cells to the GLS knockdown cells.