 Antisolvent
Precipitation in Supercritical Fluids
Antisolvent
Precipitation in Supercritical Fluids
Introduction
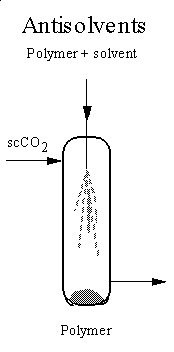
The fact that many substances are insoluble in supercritical fluids has
given rise to the use of the supercritical fluids as antisolvents
to precipitate materials from conventional solvents. The principle is similar
to the familiar use of aliphatic hydrocarbons to precipitate materials
from a more polar organic solvent. We have developed a miniature apparatus
for supercritical antisolvent precipitation which differs from other designs,
in that the apparatus is much smaller and the organic solution is injected
essentially under conditions of laminar flow. Our equipment has been applied
to particle formation in materials as diverse as polybutadiene, laser dyes
and bucky balls (C60). The key advantage is that, the size and
morphology of the particles can be manipulated by adjusting the parameters
(flow rates, pressure, temperature of CO2, etc.)
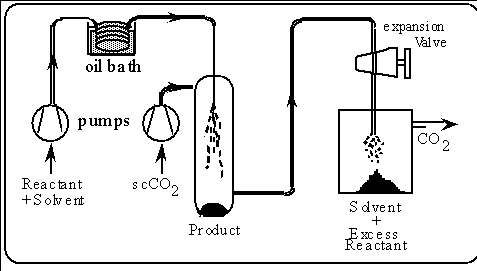
We have now combined a flow-reactor using conventional solvents with
scCO2 antisolvent precipitation for the synthesis of organometallic
compounds.
-
The reaction is carried out in an organic solvent in a thermal flow reactor
and the product is then precipitated by the scCO2 antisolvent,
as shown below.
-
The high pressure of the scCO2 (typically 10 MPa) means that
the organic solvent also has to be pumped at a pressure which is higher
than the critical pressure of most organic liquids ( e.g. pyridine, Pc
5.63 MPa, 56 bar.).
-
The result is that the organic solvent can be heated right up through its
critical temperature without boiling. Thus, it is possible to carry out
reactions in highly superheated organic solvents without the use of conventional
high pressure autoclaves.
The technique has been given the acronym ROSA, (Reaction in Organics with
Supercritical Antisolvents).
The standard routes to cis-W(CO)4(pyr)2 are relatively
time-consuming and difficult. The refluxing of W(CO)6 in pyridine
at 115 °C leads to formation of the tris product, fac-W(CO)3(pyr)3,
see Scheme. cis-W(CO)4(pyr)2 can be separated from
the mixture if the reaction is monitored continuously and is then stopped
before completion. Alternatively, cis-W(CO)4(pyr)2
can be obtained by superheating W(CO)6 in pyridine to 210 °C
in an autoclave .
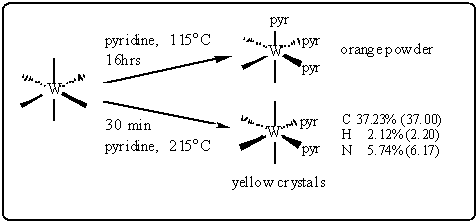
The ROSA procedure is much simpler. Pumping a 1 % (w/v) solution of
W(CO)6 in pyridine through the reactor at 215 °C with a flow rate of
100 mL/min. (i.e. with a nominal residence time of 30 min.) leads to the
precipitation of yellow crystalline needles, 2 - 3 mm long, identified
as pure cis-W(CO)4(pyr)2, see Scheme. The size of
the precipitated crystals could be varied by changing the flow rate of
scCO2; lower flow rates led to larger crystals.
Reactions currently in progress include Cr(CO)3 derivatives
of cyclophanes, which again are difficult to prepare by conventional routes.
Preliminary results have been extremely encouraging. However, we believe
that the significance of the ROSA technique is much wider because
-
the same equipment can be used for almost any organic solvent without modification;
-
the apparatus is inherently scalable and reactions could be carried out
on a much larger scale;
-
the technique need not be restricted to organometallic chemistry and a
similar approach could be applied to widely different areas of chemistry.
Further Information
For further information please contact M.
Poliakoff
Key Publications from Nottingham
-
Precipitation of Solvent-Free C60(CO2)0.95
from Conventional Solvents: a New Antisolvent Approach to Controlled Crystal
Growth using Supercritical Carbon Dioxide. C. N. Field, P. A. Hamley, J.
M. Webster, D. H. Gregory, J. J. Titman and M. Poliakoff., J. Am. Chem.
Soc. 2000 122, 2480-88.
Further Reading
-
Gallagher, P.M.; Krukonis, V.J.; VandeKielft,
L.J., Proc. 2nd Intl. Symp. on Supercritical Fluids (ed. McHugh.
M.A.) Johns Hopkins University, Baltimore, 1991, p45.
-
Dixon, D.J.; Bodmeier, R.A.; Johnston, K.P.; AIChE J., 1993, 39,
127;
-
Randolph, T.W; Randolph, A.D.; Mebes, M.; Yeung, S. Biotechnol. Prog.,
1993, 9, 429; (d) Yeo, S.-D.; Lim, G.-B.; Debenedetti, P.G.; Bernstein,
H.; Biotechnol. Bioeng., 1993, 41, 341.
University of Nottingham Welcome
page
Inorganic
Chemistry Welcome page
The Clean Technology
Research Group Welcome page
Page created by: Simon Poliakoff
Created: July 1997
Last Revised: January 2001
 Antisolvent
Precipitation in Supercritical Fluids
Antisolvent
Precipitation in Supercritical Fluids
 Antisolvent
Precipitation in Supercritical Fluids
Antisolvent
Precipitation in Supercritical Fluids


