 Organometallic
Reactions in Supercritical Fluids
Organometallic
Reactions in Supercritical Fluids
Introduction
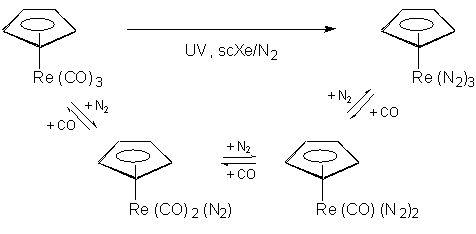
The miscibility of permanent gases with supercritical fluids is a key advantage
for organometallic chemistry. It has enabled us to generate a whole series
of new dihydrogen and dinitrogen complexes which would be difficult if
not impossible to prepare in conventional solvents.
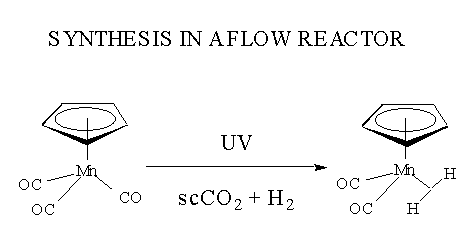
Organometallic Synthesis in a Flow Reactor
The reactions in the scheme were carried out on a small scale in a spectroscopic
cell. Supercritical flow reactors have allowed us to scale up some of the
reactions to 50 - 100 mg scale. For example, we have been able to isolate
the first dihydrogen compound of manganese.
The supercritical fluid plays several roles:
-
It provides a high concentration of H2
-
This high concentration is maintained right up to the moment when the product
is isolated, thereby minimizing the chances of product decomposition.
-
The fluid allows the product to be separated by rapid expansion of the
fluid. Such compounds have weakly bound ligands (ie H2) which
are easily lost under the vacuum which would normally be used to isolate
a product from conventional solution.
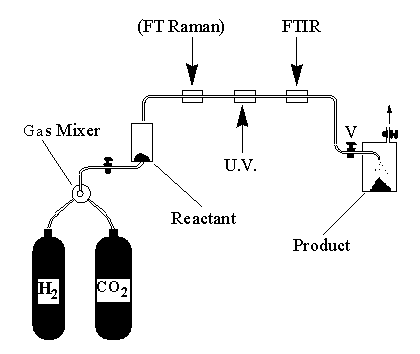
Spectroscopic monitoring is a key feature of this reactor. The FT-Raman
allows the concentation of H2 to be monitored. While the FT-IR
allows the conversion of reactant to product to be maximised by adjustment
of the reactor parameters (flow rate etc).
Recently, we have used our flow reactor techniques to generate and characterise
several other unstable compounds, such as N2O complexes and
propene complexes.
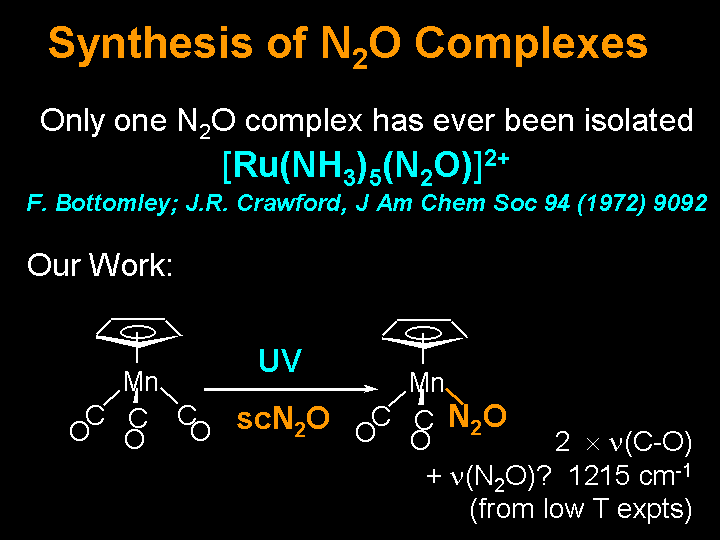
N2O Complexes
Further Information
For further information please contact M.
Poliakoff
Key Publications
-
Organometallic Synthesis as a Continuous Process: the Synthesis and the
Isolation of Cr(CO)5(h2-C3H6)
and (C5R5)Mn(CO)2(h2-C3H6)
(R = H and Me) from Superheated Liquid Propene, J. L. King, K. Molvinger
and M. Poliakoff, Organometallics, 2000, 19, 5077-82.
-
IR Evidence for the Generation of (C5H5)Mn(CO)2(N2O)
in Near-Critical N2O at Room Temperature and in Polyethylene
Matrices at Low Temperature. K. Molvinger, G. Childs, M. Roper, M. W. George
and M. Poliakoff, Chem. Letters, 2000, 11,1260.
-
New Directions in Inorganic and Metal-Organic Coordination Chemistry in
Supercritical Fluids, Jawwad A. Darr and Martyn Poliakoff, Chem. Rev.1999,
99,
495-541.
-
Clean Chemistry in Supercritical Fluids, M. Poliakoff, S. M. Howdle and
M. W. George, Process Technology Proceedings (Elsevier), 1996,
12,
67-72.
-
"Solvent-Free" Photochemical Activation of CH4, C2H4
and C2H6 by (C5Me5)Ir(CO)2
in Supercritical Fluid Solution, J.A. Banister, A.I. Cooper, S.M. Howdle,
M. Jobling and M. Poliakoff, Organometallics, 1996, 15, 1804
- 12
-
Flow Reactors for Preparative Chemistry in Supercritical Fluid Solution:-
The "Solvent-Free" Synthesis and Isolation of Cr(CO)5(C2H4)
and (h5-C5H5)Mn(CO)2(h2-H2),
(J. A. Banister, P. D. Lee and M. Poliakoff, Organometallics, 1995,
14,
3876 - 85.
-
Inorganic and Related Chemical Reactions in Supercritical Fluids, M. Poliakoff,
M.W. George and S.M. Howdle, Chapter 5 in "Chemistry under Extreme and
non-Classical Conditions, Ed. R. Van Eldik, Spektrum, Heidelberg,
1996, ISBN 0-471-16561-1, 189-218.
University of Nottingham Welcome
page
Inorganic
Chemistry Welcome page
The Clean Technology
Research Group Welcome page
Page created by: Simon Poliakoff
Created: July 1997
Last Revised: January 2001
 Organometallic
Reactions in Supercritical Fluids
Organometallic
Reactions in Supercritical Fluids
 Organometallic
Reactions in Supercritical Fluids
Organometallic
Reactions in Supercritical Fluids



