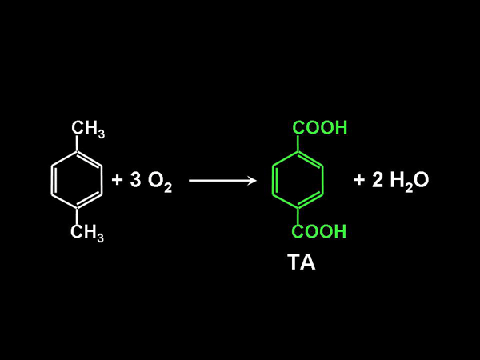
Our research in this area has recently been summarised in a review “Organic Reactions in High-Temperature and Supercritical Water” (J. Fraga-Dubreuil and M. Poliakoff) Pure & Appl. Chem. (2006) 78, 1971-82. Most of this work has been targeted on the selective partial oxidation of alkyl aromatics, particularly on p-xylene to terephthalic acid [TA]
The principal advantage of scH2O in this reaction is that TA is soluble in scH2O so that there is no co-precipitation of TA with partly oxidized intermediates. This co-precipitation is a major limitation of current commercial processes which use acetic acid as the solvent. Full details can be found in the publications;
- “Selective Partial Oxidation in Supercritical Water: the Continuous Generation of Terephthalic Acid from para-Xylene in High Yield” (P. A. Hamley, T. Ilkenhans, J. M. Webster, E. Garcia-Verdugo, E. Venardou, M. J. Clarke, R. Auerbach, W. B. Thomas, K. Whiston and M. Poliakoff, Green Chem., (2002), 4, 235-38.
- “Is it possible to achieve Highly Selective Oxidations in Supercritical Water? Aerobic Oxidation of Methylaromatics Compounds” (E. Garcia-Verdugo, E. Venardou, W. B. Thomas, K. Whiston, W. Partenheimer, P. A. Hamley and M. Poliakoff) Adv. Synth. Catal. (2004) 346, 307-16.
- “Simultaneous Continuous Partial Oxidation of Mixed Xylenes in Supercritical Water” (E. G. Verdugo, J. Fraga- Dubreuil, P. A. Hamley, W. B. Thomas, K. Whiston and M. Poliakoff, Green Chem. (2005) 7, 294-300.
- “Catalytic Selective Partial Oxidations Using O2 in Supercritical Water: the Continuous Synthesis of Carboxylic Acids” (J. Fraga-Dubreuil, E. Garcia-Verdugo, P. A. Hamley, E. M. Vaquero, L. M. Dudd, I. Pearson, D. Housley, W Partenheimer, W. B. Thomas, K. Whiston, K. and M. Poliakoff) Green Chem. (2007) 9, 1238-1245.
- "Chemical recycling of carbon fibre reinforced composites in nearcritical and supercritical water" (R. Piñero-Hernanz, C. Dodds, J. Hyde, J. García-Serna, M. Poliakoff, E. Lester, M. J. Cocero, S. Kingman, S. Pickering and K. H. Wong), Composites Part A: Applied Science and Manufacturing, (2008), 39, 454-461
Other recent studies include “The Continuous Synthesis of ε-Caprolactam from 6-Aminocapronitrile in High-Temperature Water” (C. Yan, J. Fraga-Dubreuil, E. Garcia-Verdugo, P. A. Hamley, M. Poliakoff, I. Pearson and A. S. Coote) Green Chem. (2008) 10, 98–103.
We also study a smaller number of reactions as batch processes. The advantage of scH2O s that, on cooling the reactor, some products can be crystallised so that they require no further purification. Details of the reactor can be found in “A Study of the Synthesis of Benzimidazoles in High-Temperature Water” (L. Dudd, E. Venardou, E. Garcia-Verdugo, P. Licence, A. J. Blake, C. Wilson and M. Poliakoff) Green Chem. (2003) 5, 187–192.
A more recent example is “Rapid and Clean Synthesis of Phthalimide Derivatives in High-Temperature, High-Pressure H2O/EtOH Mixtures” (J. Fraga-Dubreuil, G. Comak, A. W. Taylor and M. Poliakoff) Green Chem.,(2007) 9, 1067-1072.
|
We always welcome e- mails to martyn.poliakoff@nottingham.ac.uk from those interested in this area or who would like reprints of papers.
