About ESIP
ESIP has been developed for use by national and local decision makers, and researchers. It can be used to extend results of within-pregnancy trials to a lifetime perspective, as well as evaluating interventions on a national scale. Currently it is configured to model estimates which are relevant to the UK only, but it could be tailored to evaluate tobacco cessation interventions delivered to pregnant women in other countries.
How was ESIP constructed and what does it include?
ESIP was constructed after an extensive reviews of both the economic and epidemiological literature on the risks and benefit smoking in pregnancy and is written in Microsoft Excel to allow easy access. It includes most morbidities for the mother and infant that are associated with maternal smoking in pregnancy, which are:
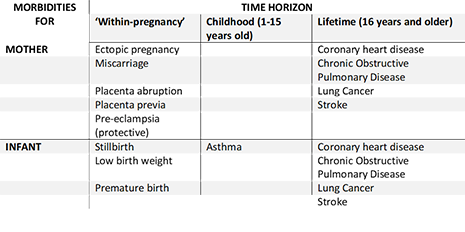
Furthermore, ESIP estimates the impact of maternal smoking on the uptake of smoking amongst their offspring.
See more information on the development of ESIP.
What output can ESIP generate?
ESIP estimates several value for money statistics, with the primary measures of cost-effectiveness being incremental cost-effectiveness ratio per additional quality adjusted life year. Secondary cost-effectiveness measures include incremental cost-effectiveness ratios per:
-
Additional life year
-
Additional quitter
-
Per maternal ‘within-pregnancy’ morbidity avoided (ectopic pregnancy, miscarriage, placenta abruption, placenta previa, and pre-eclampsia)
-
Per infant ‘within-pregnancy’ morbidity avoided (stillbirth, low birth weight, premature birth)
These ratios can be generated for both mother and infant separately, or combined into one single statistic, using lifetime or end-of-pregnancy horizons.
ESIP can also be used to estimate the return on investment of an intervention compared to a comparator. We defined this as the ratio of the healthcare cost savings generated by the intervention divided by the increased cost of the intervention.
ESIP generates a series of benefit-cost ratios for mother and infant, using lifetime or end-of-pregnancy horizons.
Output includes both deterministic analyses based on point estimates of model inputs, and probabilistic sensitivity analyses using fitted distributions.
Why use ESIP?
ESIP has some key advantages over previously-developed models:
- A link that allows maternal smoking to directly impact on infant’s health and their smoking uptake.
- A time horizon which includes all sufficiently impactful short and long-term morbidities and outcomes for both mother and infant.
- Includes a robust approach to identifying morbidities for inclusion.
- Allows for greater uncertainty in model parameters than previous models.
Can I see ESIP myself?
Download an Excel file which demonstrates a deterministic analysis conducted in ESIP. Some inputs can be changed (e.g. quit rates, intervention costs), however most parameters cannot.
If you are unsure whether ESIP is suitable for you, you may want to download this Excel file to investigate the estimates ESIP can generate.
*You may download and view this file for your personal non-commercial use, subject to Copyright and the Terms and Conditions below.
How can I use ESIP?
For UK researchers: ESIP is already setup to evaluate smoking cessation interventions within the UK. If you wish to use ESIP to evaluate such an intervention, then please contact the project lead, Dr Matthew Jones at:
E-mail: matthew.jones3@nottingham.ac.uk
Tel: 0115 74 86710
For international researchers
For international researchers: Current parameters for ESIP come from UK based data, and therefore may not be relevant to your country. However, it may be possible to use ESIP to evaluate cessation interventions in other countries if suitable data can be found to parameterise ESIP to be more suited to the country in question. For more information, please contact the project lead, Dr Matthew Jones at:
E-mail: matthew.jones3@nottingham.ac.uk
Tel: 0115 74 86710
Pubications using ESIP