Molecular frame photoelectron angular distribution
Written by: Dhirendra Singh
January 30, 2018
Background
Angle-resolved photoelectron spectroscopy is a sensitive technique that enables detailed information on the dynamics of photoionization of gas phase molecules. In order to maximize the information extracted from angular distributions it is desirable to measure them relative to the molecular frame of the molecules rather than to the laboratory frame [1]. In weak field limit such measurement can provide ways of obtaining complete information of photoionization dynamics, which mean all the radial dipole matrix elements, and the phase that governs the dynamics, can be determined directly from experiments [2, 3] and further information on molecular orbitals can be extracted from these phases. Radial dipole matrix elements can also be determined from a carefully designed experiment [4, 5] with the single exception of phase difference between even and odd partial waves, whose interference give rise to an up down symmetry in a measured PAD [6].
Molecular frame photoelectron angular distribution (MF-PAD) have been used to perform the examination of quantum mechanical phenomenon [7] and provide an extremely useful method to study the continuum resonance both in ionization and dissociation [8]. It has also been shown that MF-PADs provide direct information on molecular orbitals if the ionization in the tunneling regime. So achieving MF-PADs is extremely useful for fundamental studies.
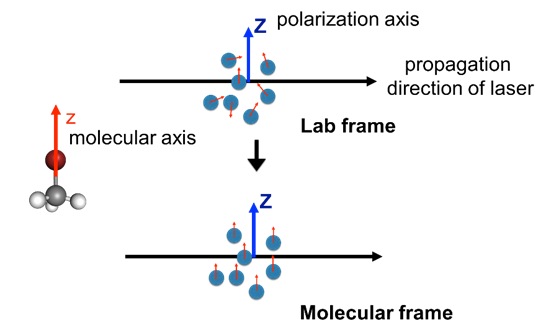
Fig. 1: Molecular frame and Lab frame
One method of accessing these MF-PADs involves aligning the molecules by interaction with a laser pulse prior to the photoionization. For example, it is possible to align an ensemble of symmetric top molecules using resonant steady state excitation with polarized light; this causes the molecular symmetry axes to have a well characterized ensemble alignment in space [9].
An alternative is to use non-resonant polarized laser light to create alignment in the molecule. In past few years this technique has been extended to the alignment of polyatomic molecules in three dimension as well [10]. An advantage of this technique over others is that it is not inherently limited to small molecular systems only. This is because the interaction depends on the polarizability of the molecule; the laser field induces a maximum dipole moment along the most polarizable axis which then aligns with the axis of the laser field [11].
Our research:
Our aim is to characterize the photoionization dynamics of polyatomic molecules such as aniline, methyl iodide, di-iodobenzene, benzonitrile, and methyl oxirane using the technique of velocity map imaging. To achieve this we will implement the strong field alignment technique at University of Nottingham. So far we have been working on PADs model calculations using the formalism provided in the paper of Underwood and Reid [12]. These model LF PADs calculations have been done using alignment coefficients that are achievable in cold beams of CH3I and p-di-iodobenzene, using a 1.3 ps laser pulse. LF-PAD similar to the MF- PAD has been seen when degree of alignment increases. This information about the effect of specific alignments opens the door to perform the experiment with the similar condition to measure the MF-PAD.
Some preliminary results:
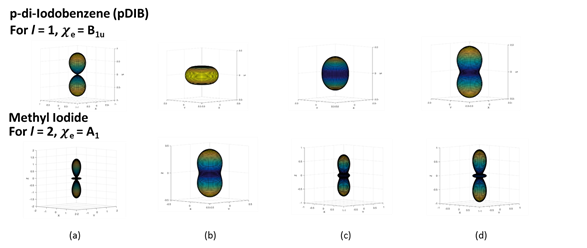
Fig. 2: Photoelectron angular distributions for model cases. (a) MFPADs, (b) LFPADs from an unaligned distribution, (c) LFPADs from a distribution that has not been well aligned, ⟨cos2θ⟩ = 0.664 for MI (rotational temperature 10 k, laser intensity 1 TW/cm2) and ⟨cos2θ⟩ = 0.618 for pDIB (rotational temperature 5 k, laser intensity 1 TW/cm2), (d) LFPADs from a distribution that has been well aligned ⟨cos2θ⟩ = 0.825 for MI and ⟨cos2θ⟩ = 0.778 for pDIB with a 1.3 ps laser pulse, rotational temperature is 5 K and laser intensity 10 TW/cm2.
Personal experience as an early stage researcher (ESR):
Working in the network of different labs all over the Europe is the greatest the opportunity one can get. It teaches an ESR how to work as a community in the field of science and progress by sharing knowledge, expertise and techniques. Moreover, the secondment opportunities afforded to me as an ESR allow me to discuss my research with other colleagues and receive training at the host institutes. This, in turn, broadens my research horizons and provides new perspectives to enable me to tackle problems that we face undertaking our research.
References:
|
[1]
|
K. L. Reid, Ann. Rev. Phys. Chem., vol. 54, pp. 397-424, 2003.
|
|
[2]
|
M. Lebech, J.C. houver, A. Lafosse, D. Dowek, C. Alcaraz, L. Nahon and R.R. Lucchese,, J. Chem. Phys., vol. 118, p. 9653, 2003.
|
|
[3]
|
T. Teramoto, J. Adachi, K. Hosaka, M. Yamazaki, K. Yamanouchi, N.A. Cherepkov, M. Stener, P. Decleva and A. Yagishita, J. Chem. Phys. B, vol. 40, p. F241, 2007.
|
|
[4]
|
P. Hockett and K. L. Reid, J. Chem. Phys. 127, 154308, 2007.
|
|
[5]
|
P. Hockett, M. Staniforth, K. L. Reid and D. Townsend, Phys. Rev. Lett. 102, 253002, 2009.
|
|
[6]
|
K. L. Reid, Mol. Physics, 110, 3, 2012.
|
|
[7]
|
D. Akoury, K. Kreidi, T. Jahnke, T. Weber, A. Staudte, A. Czasch, O. Jagutzki, R.A.C. Fraga, R.E. Grisenti, P. Ranitovic, C.L. Cocke, T. Osipov, H. Adaniya, A.L. Landers, H. Schmidt-Bocking and R. Dorner, Science, 318, 949, 2007.
|
|
[8]
|
O. Gessner, A.M.D. Lee, J.P. Shaffer, H. Reisler, S.V. Levchenko, A.I. Krylov, J.G. Underwood, H. Shi, A.L.L. East, D.M. Wardlaw, E.T. Chrysostom, C.C. Hayden and A. Stolow, Science, 311, 219, 2006.
|
|
[9]
|
Thomas A Field, Katharine L. Reid, J. Chem. Phys., vol. 111, no. 4, 1999.
|
|
[10]
|
J.L. Hansen, H. Stapelfeldt, D. Dimitrovski, M. Abusamha, C.P.J. Martiny and L.B. Madsen, Phys. Rev. Lett. 106, 073001, 2011.
|
|
[11]
|
Tamar Seideman, Henrik Stapelfeldt , Review of Modern Physics, vol. 75, 2003.
|
|
[12]
|
J. G. Underwood and K. L. Reid, J. Chem. Phys., 113, 3, 2000.
|