Valence shell photoelectron circular dichroism of chiral amino-acids and implication in the homochirality of life
Written by: Rim Hadidi
February 28, 2019
Background
Photoelectron circular dichroism (PECD) is a chiroptical effect which has been studied for the past 10 years as a probe to understand molecular chirality (see Hassan’s blog). When circularly polarised light (CPL) is used to ionise randomly oriented pure enantiomers of a chiral molecule, PECD can be visualised as a forward/ backward asymmetry in the photoelectron angular distribution (PAD) with respect to the light’s propagation’s axis.
One of the unresolved mysteries in science is the origin of homochirality of chiral molecules in living organisms. In other words how did life become handed? For example amino-acids are all left handed (L-enantiomer), with very few exceptions, and the sugars in the RNA/DNA are right handed (D-enantiomers).
Amino acids, the building blocks of proteins, are chiral and may provide a clear dichroic signature when probed with a chiroptical technique such as PECD. PECD asymmetry may reach several tens of %, that is, orders of magnitude above classical circular dichroism (CD), providing a more sensitive technique to study molecules of this sort.
Amino acids were discovered in carbonaceous meteorites with an enantiomeric excesses (ee) and isotopic composition, indicating an extra-terrestrial origin. They were also synthesised in the laboratory by far-UV irradiation of interstellar/circumstellar medium (ISM/CSM) [4] ice analogs.
Proline (Pro) is an amino acid that is relevant in astrochemistry since it belongs to the first five amino acids to have been recruited into the genetic code [5] and has been detected in the Murchison meteorite in large quantity with an L- excess [6]. Here we report electron imaging measurements made at the Lyman-α radiation photon energy (10.2 eV) of two amino-acids (aa) Alanine (Ala) and Pro. Both of these amino acids are relevant to astrochemistry as they belong to the first five amino acids to have been recruited into the genetic code. Additionally, both reveal a strong overall asymmetry for the outermost orbital.
PECD can help explain the origin of biomolecular asymmetry by showing a noticeable enantiomeric excess (ee) when acting on gas-phase amino acids and could be used an alternative photophysical asymmetric process.
Experimental results
In order to take PECD measurements of amino acids, they must be vaporised. However, due to their very low vapour pressure and thermos-labile character, amino acids are quite challenging to bring into the gas phase. Therefore we used and benchmarked two complementary vaporisation methods:
- Resistive heating (RH) in an oven, followed by a supersonic molecular beam expansion, and
- Aerosol thermodesorption (TD).
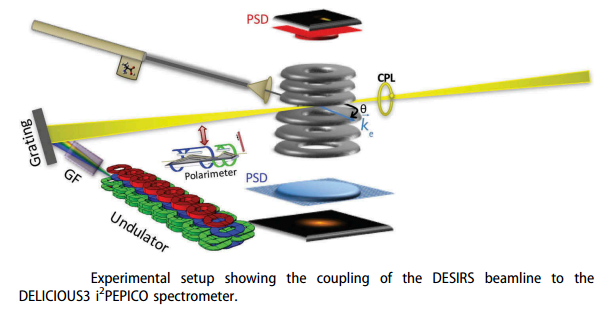
Figure 1: [2]
Two studies, Tia et al. and Hadidi et al., conducted in the SPHIRS chamber with the DESIRS beamline at Synchrotron-soliel (France) recorded PECD over a large vacuum ultraviolet (VUV) range using a (i2PEPICO) spectrometer DELICIOUS3. Note that a new high-throughput aerodynamic lens was used for Proline (Figure 1).
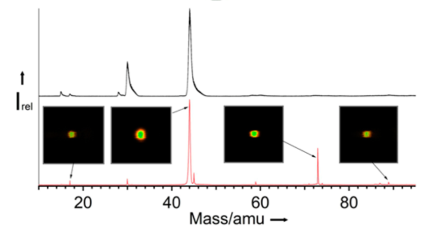
Figure 2: Time-of-flight (TOF) spectra of alanine photoionised at 10.2eV with both methods TD and RH. [1]
With both vaporisation methods, one-photon ionisation was accomplished by photoionising the gaseous neutral Ala (Pro) molecules with VUV CPL from a variable polarisation Synchrotron beamline. PECD was measured at fixed photon energies by alternating right and left circularly polarised light according to our usual method, see Nahon et al. [3], on both L and D enantiomers of Ala (Pro) over a large photon energy range. The corresponding electron and ion images, detected in coincidence, are obtained via a double-imaging photoelectron-photoion coincidence (PEPICO) spectrometer, as represented schematically in Figure 1. For a given enantiomer, photoelectron images are recorded for any chosen peak in the mass spectrum by alternating RCP (right-handed circular polarisation) and LCP (left-handed circular polarisation) light.
The two photoelectron images can be added or subtracted to tell us different characteristics of the amino acids. The difference (LCP-RCP) image yields the chiroptical parameter b1 for a given electron kinetic energy which reverses sign when we switch the enantiomer (Figure 3-a). The sum (LCP+RCP) image provides the corresponding photoelectron spectrum (PES) which shows the resolved bands for several valence orbital contributions of the chiral molecule and the anisotropy parameter b2. Both TOF (Time Of Flight) spectra of Ala from (Figure 2) are dominated by the fragment m/z 44, corresponding to the loss of -COOH, as reported in previous work. The one obtained by resistive heating (red peaks) shows additional peaks (m/z 17, 59, 73). These ions are as cold as the parent molecule, as inferred from their TOF peak width correspond to spurious contributions, due to thermal decomposition of Ala in the oven prior to the supersonic expansion.
For the oven vaporisation method, the raw difference images obtained at Lyman-α radiation energy for electrons correlated to Ala-related ions (m/z 44, 45, and 89) as selected via the PECD-PICO scheme.
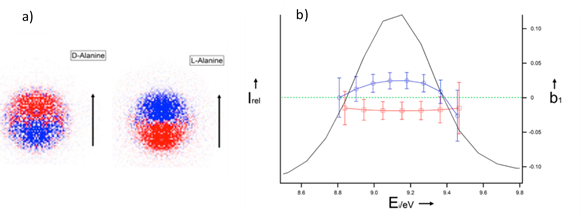
Figure 3: a) Raw difference images, filtered on all alanine-related ions, showing a PECD-induced forward-backward asymmetry with respect to the light-propagation axis (depicted by the arrow). b) Parent-filtered (m/z 89) PECD and PES measurement recorded at 10.2 eV on oven-produced D-(blue curve) and L-alanine (red curve). The average b1 value of 0.02 corresponds to a 4% asymmetry. [1]
A clear PECD induced forward-backward asymmetry of about 4% with respect to the light axis is visible on the parent-filtered cation Ala, with a sign reversing upon switching the enantiomer (Figure 3). The analysis of these images leads to the PES and b1 distribution over the HOMO electronic band, or highest occupied molecular orbital, as seen in Figure 3-a.
Another striking result was found with Pro at the Lyman-α energy. The b1 dichroic parameter associated with the Pro parent possesses a PES-averaged absolute value of 0.06, corresponding to a PECD asymmetry of 12%, i.e. three times larger than the corresponding result on Ala and interestingly of the same sign (Figure 4).
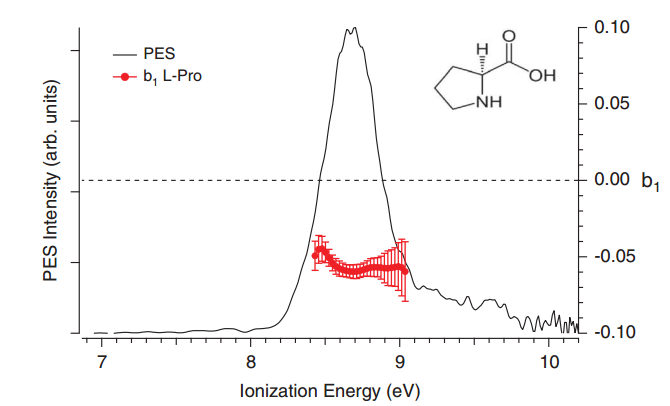
Figure 4: PECD of L-proline at Lyman-α radiation (10.2 eV).
Notes: Parent-filtered (m/z 115) PES and b1 measurements on TD-produced L-proline (red curve).[2]
Implication in the origin of biomolecular homochirality
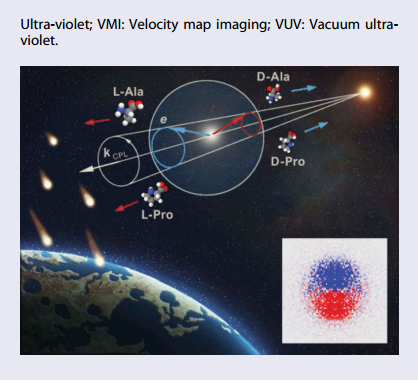
Figure 4: [2]
Thinking again about the chirality of biological molecules, let us consider that gas-phase Ala (Pro) is present in the ISM/CSM as racemate or mixture of both L and D forms (Figure 4).
If this racemic mixture of gas-phase Ala (Pro) is embedded into a partial CPL field with a given and constant helicity over a large region of space, similar to the one from which the Sun was formed discussed in [7], and is irradiated with VUV-CPL defining an orientation in space; then b1 is anti-symmetric with the swapping of enantiomers. This means that the PECD induced electron asymmetry at the astrophysically relevant energy ‘Lyman-α’ radiation (10.2 eV) will be opposite for the two enantiomers.
Following the principle of momentum conservation, the corresponding recoiling ions will also have opposite asymmetries, i.e.opposite directions of propagation. This process therefore leads to an asymmetry in the flux of non-fragmented Ala (Pro) parent ions in a given line of sight, which may reach a maximum value of 2b1in the limit of pure CPL.
In other words, along the photon axis direction, the expanding sphere of recoiling ions presents an asymmetry of up to 4% (12%) for Ala (Pro), which corresponds to a net enantiomeric excess (e.e.) of the same value. This enantio-enriched gas phase Ala (Pro) ion cloud of a given handedness, separating from its enantio opposite counterpart, may then be captured, neutralised, and embedded into comets and meteorites seeding Earth with an exogenous organic matter presenting an initial e.e.
Besides the striking intensity for Pro compared to Ala, which may lead in a given line of sight to a 12% e.e. of the recoiling parent Pro ion, the crucial point is that at Ly-α, the b1 parameter for Pro is of the same sign as the one for Ala, leading to an associated e.e. of the same sign, which strengthens our hypothesis of a possible link of PECD to the origin of life’s homochirality.
Summary
PECD could be an alternative photophysical asymmetric process, acting on gas-phase amino acids, which could lead to noticeable ee.
We collected data on the full VUV photodynamics and chiroptical behaviour upon ionisation of Pro. We came up with a rich array of mass spectra, TPEPICO scans, PES and PECD measurement over a large photon energy range.
After the study of the simplest chiral amino acid, alanine, the result on Pro strengthens the hypothesis of a possible link of PECD with the origin of life homochirality. This is based upon an enantio-enrichment of the amino acids parent ion in a given line of sight due to the asymmetric recoil of the ions induced by the asymmetric emission of the corresponding photoelectrons (momentum conservation).
The results at the lyman α radiation strengthens the astrobiological scenario proposing a link between PECD and the origin of life’s homochirality.
All of this is strengthened and benchmarked with numerical simulations.
This work is extended to the corresponding homochiral chiral nanoparticles in order to observe a possible amplification of the PECD-induced asymmetry by a local order effect as compared to the free molecule or at the opposite to observe if the initial photon polarisation memory is lost because of internal electron inelastic scattering.
Experience as an ESR
As an ESR, and within the context of ASPIRE, I gained scientific knowledge not only at my own institution but at collaborating laboratories across the world through secondments. I have gained broad perspectives in different areas of science allowing me to be versatile and a risk taker in many areas from academia to industry. I gave talks and presented posters at conferences and seminars that I attended within the training context. These experiences allowed me to improve my communication and scientific skills, and have helped me gain confidence in myself as a scientist.
References
[1] M. Tia, B. C. de Miranda, S. Daly, F. Gaie-Levrel, G. A. Garcia, I. Powis, and L. Nahon, J. Phys. Chem. Lett. 4, 2698 (2013).
[2] R. Hadidi, D. K. Bozanic, G. A. Garcia, and L. Nahon, Adv. Phys. X (2018).
[3] L. Nahon, G. A. Garcia, C. J. Harding, E. A. Mikajlo, and I. Powis, J. Chem. Phys. 125, 114309 (2006).
[4] U. J. Meierhenrich, I. Myrgorodska, C. Meinert, Z. Martins, L. Le Sergeant, and U. J. Meierhenrich, 1402 (2015).
[5] I. King Jordan, F. Kondrashov, I. Adzhubel, Y. Wolf, E. Koonin, A. Kondrashov, and S. Sunyaev, Nature 433, 633 (2005).
[6] M. H. Engel and S. A. Macko, Nature 389, 265 (1997).
[7] C. Meinert, P. de Marcellus, L. L. d’Hendecourt, L. Nahon, N. C. Jones, S. V Hoffmann, J. H. Bredehoft, and U. J. Meierhenrich, Phys. Life Rev. 8, 307 (2011).