N-READY Workstreams
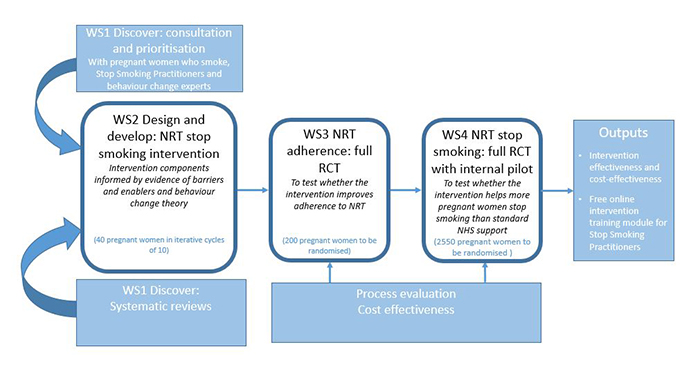
During this workstream, we carried out background research to discover what makes it easier and harder for pregnant women to use NRT. The research team looked at published research to help summarise the latest evidence on NRT safety, nicotine exposure from NRT and factors that influence NRT use in pregnancy. The team also worked with pregnant women, stop smoking practitioners, public contributors and other experts to identify what can be done to positively change what pregnant women think (beliefs) about NRT and what they do (behaviour) when using it. Using this evidence alongside health behaviour theory, the team developed content for the intervention. This included a variety of new messages and approaches to motivate and support pregnant women to use enough NRT. We then spoke with pregnant women and stop smoking practitioners to examine the acceptability of these ideas with pregnant women and stop smoking specialists.
See the Workstream 1 study protocol which describes what we did.
The findings generated from this work can be found here [See N-Ready Publications]
Workstream 2: Design and develop
Using the findings from workstream 1, the research team designed a prototype NRT support package. This was then improved by testing it on three groups of pregnant women who wanted to quit smoking, one after another. A total of 35 pregnant women took part in a pre-quit consultation and were offered the support package for 28 days from their quit date, and the asked to provide feedback. Alongside this, participants tested mobile phone app designed to measure how much NRT they had used each day and a questionnaire which measures positive and negative thoughts about NRT.
See Workstream 2 protocol
The results showed that pregnant women liked the intervention and new research measures. Findings are now being written up for publication and wider dissemination.
Workstream 3: Test NRT adherence
This is a multi-location trial called SNAP 2, which aims to see whether the new support package helps to improve use of NRT in pregnant women, while monitoring how much nicotine they receive. Recruitment began in July 2021 and is expected to last 12 months. Click here for further information about SNAP 2.
Alongside the trial, the research team will explore whether the support package has been delivered as planned, identify how it might be delivered in routine care, and monitor the delivery costs.
Workstream 4: Test stop smoking
In this workstream the research team will test whether the NRT support package helps more pregnant women to quit smoking than usual support.
Before starting this second randomised control trial, the National Institute for Health Research (NIHR) will review the results of the first trial to ensure that further investigation is warranted and that a large-scale trial is possible.
The primary outcome will be to see how many pregnant women can remain smoke free for at least 6 months. A cost-effectiveness analysis of the support package will be carried out. A ‘fidelity checklist’ will be used to assess the extent to which the intervention is delivered as planned and to identify any obstacles that may have affected delivery.
Back to study homepage