Tuesday, 14 May 2019
A landmark clinical trial is aiming to improve the prevention of potentially life-threatening infection caused by group B Streptococcus in newborn babies in the UK.
The £2.8 million NIHR-funded study will measure the effectiveness of two tests to identify group B Strep bacteria in late pregnancy or labour compared with the current approach of identifying pregnant women with ‘risk factors’ for their newborn developing the infection.
The trial will involve 80 hospitals in England, Wales and Scotland and the results will inform future pregnancy care policy in the UK.
A serious infection
Group B Strep is the most common cause of life-threatening infection in newborn babies, causing a range of serious infections including pneumonia, meningitis and sepsis (blood infection).
The UK currently does not routinely test pregnant women for group B Strep, and uses a ‘risk-based’ prevention strategy. There is ongoing debate as to which prevention strategy would be better for the UK. This ground-breaking trial should resolve this debate.
The bacteria are present in approximately one in five pregnant women, usually causing no harm to the carrier, but may be passed unknowingly from a mother to her baby around birth. In the vast majority of cases babies will be unaffected, but one in 1750 newborn babies will develop a group B Strep infection. One in 19 of these babies will die and one in 14 survivors will be left with long-term disability. It can be especially dangerous to babies born preterm.
 Daisey-May
Daisey-May
Group B Strep infections in newborn babies can usually be prevented by giving antibiotics (usually penicillin) through a vein to women during labour, which reduces the risk by up to 90 per cent.
Since 2000, there has been a rise of almost a third (31 per cent) in the rate of group B Strep infections in babies under three months of age, despite ‘risk-based’ prevention guidelines being introduced in 2003. Research has shown that the current approach is not very accurate. 65 per cent of UK newborn babies who develop group B Strep infections have mothers who had no risk factors and 70 per cent of women who do have risk factors do not actually carry the bacteria and are therefore unnecessarily given antibiotics.
Which test is best?
The trial will look at the effectiveness of two different tests – a lab-based test at 35 to 37 weeks of pregnancy, compared with a ‘bedside test’ at the start of labour. It is known that some women who test positive for group B Strep in pregnancy will not still carry the bacteria by the time they give birth.
The work is being led by Dr Kate Walker, Clinical Assistant Professor of Obstetrics, and Professor Jane Daniels, Professor of Clinical Trials at the University of Nottingham’s School of Medicine, and includes researchers from the Universities of Nottingham, Warwick, Lancaster and City of London, and the charities, National Childbirth Trust (NCT) and Group B Strep Support.
At the moment, we’re in a situation where we’re missing lots of babies whose mums don’t have risk factors but do carry the bacteria and giving antibiotics to women who don’t carry the bacteria.
We want to answer the question for the NHS — should you test pregnant women for group B Strep or not, and if you’re going to test, is it better to do a culture test at 35 to 37 weeks pregnancy or a bedside test? Hopefully the trial will answer these questions. We believe that, if testing is proved effective, this would mean that the right women get the right antibiotics.
Campaign of a lifetime
The charity, Group B Strep Support, has been campaigning for better prevention of this infection for over 20 years. Founder and Chief Executive, Jane Plumb MBE, said: “The current UK policy on group B Strep is not working. The number of babies suffering group B Strep infections has risen not fallen despite the introduction of the risk-based prevention strategy in 2003.
 Jane Plumb, Group B Strep Support
Jane Plumb, Group B Strep Support
“After routine testing was introduced in the United States, the rate dropped by over 80 per cent and their rate of early-onset GBS infections (those in the first six days of life) is now less than half that of the UK. If the rate was reduced in the UK in the same way, we could prevent group B Strep infections in approximately 350 babies every year, saving 15 babies’ lives and protecting another 15 from life-changing disability. The results of this important trial will drive improvements in UK policy and lead to fewer babies and their families suffering the trauma that group B Strep infection can bring,”
The NIHR Nottingham GBS-3 Trial is the first worldwide to compare two types of testing versus a risk-factor approach and as such could lead to improvements in pregnancy care internationally.
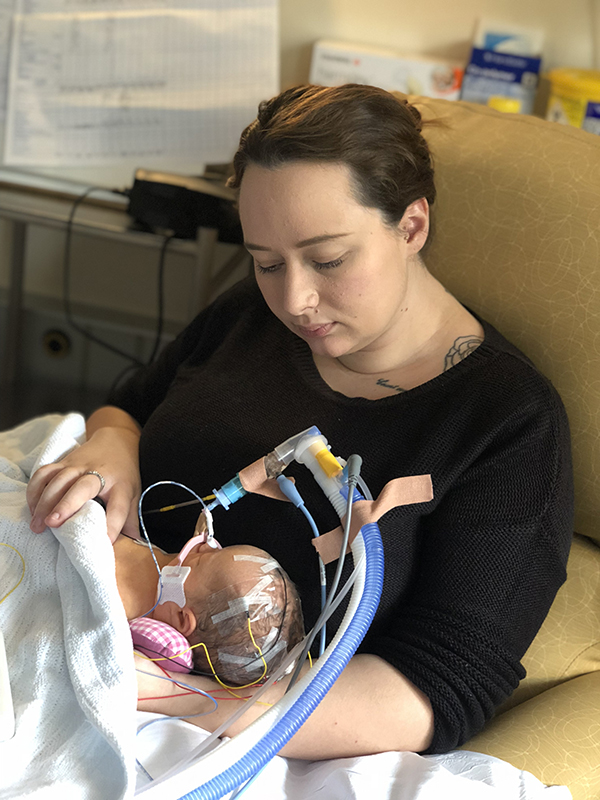 Bethany Foss and baby Daisey-May
Bethany Foss and baby Daisey-May
Case Study: Bethany Foss, from Exeter.
Bethany Foss had not heard of group B Strep when she was admitted to the Royal Devon and Exeter Hospital to give birth. Bethany believes her daughter’s future would have been very different if she had been tested for group B Strep.
Bethany’s daughter, Daisey-May Moore, was born on 30 October 2018 at 38 weeks, weighing 5lb 6oz. It soon became clear that she was seriously ill.
Bethany said: “Daisey-May seemed absolutely fine at first and was with me on the ward, but after 24 hours I noticed that she was grunting when she breathed. Soon after, I was told she had group B Strep sepsis and meningitis and to prepare for the worst. It was the worst time of our lives. We were in total shock and disbelief as to how our baby could have been born so normal and perfect, then in the space of 24 hours be so near to death.
“We ended up being moved to a different hospital, which was better equipped, but that was hard for us as we were then even further away from our other two children (Noah, 3, and Leo, 2). Every day felt like a miracle and eventually they stabilised Daisey-May enough to take her off her ventilator and to everyone’s amazement she started breathing.
“We know from an MRI scan that Daisey-May’s brain has been very badly damaged. We don’t know yet how she will be affected in the future and what she will and won’t be able to do. She also has epilepsy and is on three different medications to control her seizures,” Bethany continued.
Daisey-May was in hospital for three weeks altogether, coming home on 25 November 2018, where she is now being looked after by her parents with the help of specialist care.
 Daisey-May in hospital
Daisey-May in hospital
“We’re grateful for every day with Daisey-May, and everything she does is amazing, but we’d not heard of group B Strep until Daisey-May’s birth, despite her being our third child. I was never offered information or a test for group B Strep when I was pregnant, whereas this would have been done automatically in most developed countries.
“The whole experience has been highly traumatic. Nobody should have to have discussions about their new baby’s life when the infection they’re suffering from could so easily have been prevented,” continued Bethany Foss.
Bethany is supported by the Group B Strep Support charity (www.gbss.org.uk), which is working to eradicate the infection in babies.
Story credits
More information is available from Dr Kate Walker via email kate.walker@nottingham.ac.uk , or Emma Rayner, Media Relations Manager for the Faculty of Medicine and Health Sciences.
Notes to editors:
About the University of Nottingham
Ranked 97 in the world and 17th in the UK by the QS World University Rankings, the University of Nottingham is a founding member of Russell Group of research-intensive universities. Studying at the University of Nottingham is a life-changing experience, and we pride ourselves on unlocking the potential of our students. We have a pioneering spirit, expressed in the vision of our founder Sir Jesse Boot, which has seen us lead the way in establishing campuses in China and Malaysia - part of a globally connected network of education, research and industrial engagement.
Nottingham was crowned Sports University of the Year by The Times and Sunday Times Good University Guide 2024 – the third time it has been given the honour since 2018 – and by the Daily Mail University Guide 2024.
The university is among the best universities in the UK for the strength of our research, positioned seventh for research power in the UK according to REF 2021. The birthplace of discoveries such as MRI and ibuprofen, our innovations transform lives and tackle global problems such as sustainable food supplies, ending modern slavery, developing greener transport, and reducing reliance on fossil fuels.
The university is a major employer and industry partner - locally and globally - and our graduates are the third most targeted by the UK's top employers, according to The Graduate Market in 2024 report by High Fliers Research. Alongside Nottingham Trent University, we lead the Universities for Nottingham initiative, a pioneering collaboration between the city’s two world-class institutions to improve levels of prosperity, opportunity, sustainability, health and wellbeing for residents in the city and region we are proud to call home. More news…