Molecular Immunology of human Schistosomiasis and Echinococcosis
Schistosomes are parasitic flatworms infecting more than 200 Million people worldwide. There is currently no vaccination (although one candidate vaccine has reached advanced clinical trial stage) and only one drug, Praziquantel, is widely used against this parasite. Drug treatment does not work against all life stages and does not protect against reinfection.
Another problem afflicting countries striving to control schistosomiasis is the lack of a sensitive diagnostic technique. The widely used Kato-Katz technique (counting eggs from a defined amount of stool) is fastidious, requires trained personnel and is notoriously insensitive in areas with low prevalence and individuals with low worm loads. Tests based on detection of worm antigens are not always sufficiently specific, due to antigenic cross-reactivity with other parasites, and tests aimed at measuring anti-parasite antibodies in blood do not have the ability to differentiate past from active infection. None of the currently available methods meet all of the ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid (Robust), Equipment-free and Deliverable to end-users) for point of care tests. Hence there is a need to develop new tests which can be used for schistosomiasis control and mapping. Such antigens are yet to be discovered.
Much of the immunological schistosomiasis research published in the last decades has relied on the use of complex somatic extracts from eggs or adult worms (SEA, SWAP, etc.) or tegument extracts. Measuring global immune responses to complex antigenic mixtures rather than to individual, defined components may mask properties of individual antigens which may have a superior performance as diagnostic or vaccine candidate antigens. In particular, carbohydrates expressed on schistosomes can be highly cross-reactive and may not be related to host protective mechanisms Eberl et al., 2001.
Our approach is to express hundreds (and in the future hopefully thousands) of individual, unglycosylated antigens using cell free expression systems (e.g. wheat germ lysate), with stringent quality controls, and study the immune response to these individual antigens using microarrays and cellular assays.
Identification of ‘archetypal’ allergens in parasitic helminths
We have developed several humanised reporter cell lines (see previous section) which can report the crosslinking of FcεRI-bound IgE by certain lectins, anti-IgE antibodies or specific allergens. Using these reporter cell lines, we are able to screen large numbers of antigens against sera of individuals infected with schistosomes. This technique can be applied to any other parasite which induces Th2 biased responses in the host, and in addition to Schistosomiasis, we are currently exploring this option as a novel diagnostic approach for Echinococcosis. Our intention is to identify novel allergens in helminth parasites. The basic reason for this approach is that we believe that the IgE arm of the immune response has evolved in higher animals mainly to protect against metazoan parasites which are too large to be phagocytosed. Our hypothesis is that IgE responses against parasitic allergens represent an ‘archetypal’ immune response and that most if not all IgE responses to harmless antigens (pollen, dust mite, food allergens) can be explained as the result of ‘misrecognition’ by the adaptive immune system as parasitic antigens due to structural similarities. Such a view is at least in part supported by the notion that while there is no need to mount an immune response to harmless allergens e.g. in grass pollen, the IgE response to helminthic parasites is frequently associated with resistance to infection or post-treatment reinfection.
Using our reporter cell lines and a cell-free rapid translation system, we have developed a workflow suitable for high throughput analysis of the worm ‘allergome’.
Role of the nuclear localisation signal (NLS) of IPSE/alpha-1
IPSE/alpha-1 is a glycoprotein secreted by the egg stage of Schistosoma mansoni. It is produced by mature eggs in the IPSE/alpha-1 are made only in the endoplasmic reticulum and ribosome-rich van Lichtenberg envelope and secreted into host tissue. The primary protein sequence possesses a classical N-terminal secretory signal sequence (CSS) and an N-terminal nuclear localisation signal (NLS).
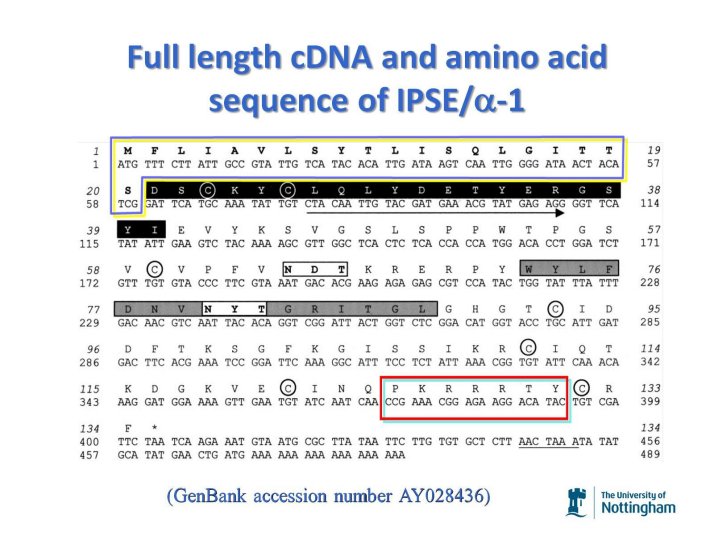
Full length cDNA and amino acid sequence of IPSE. (GenBankTM accession number (AY028436). The first 60 nucleotides at the 5’ end encode for a CSS (boxed in blue and yellow) and a putative NLS motif at the C-terminal (boxed in red and blue). Modified from Schramm et al. 2003.
Such dual occurrence of CSS and NLS on the same protein is rare. This suggested that the NLS in IPSE/alpha-1 may be functional in host cells rather than the schistosome eggs. Bearing in mind the general protein translation mechanism, it can be expected that during translation, IPSE/alpha-1 will already be targeted for secretion even before the C-terminal NLS is translated.
Ishwinder Kaur, a former PhD student in our lab, was able to demonstrate uptake of IPSE/alpha-1 by mammalian host cells and its rapid translocation to the nucleus (Kaur et al., 2011)
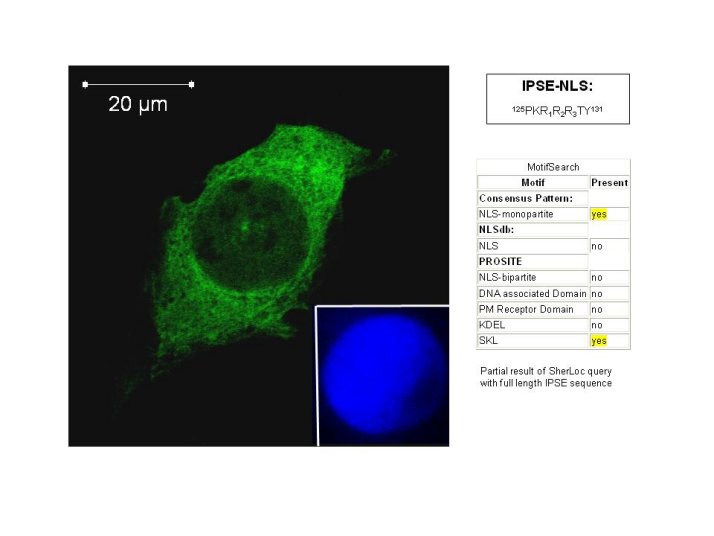
Confocal microscopy of a mammalian cell transfected with a Tetra-EGFP construct (created by (Beetz et al. 2004), containing an IPSE/alpha-1 NLS in which the wildtype PKRRRTY NLS has been mutated to PARRRTY. Substitution of any of the positively charged residue in the PKRRRTY NLS leads to complete disruption of nuclear localisation of the Tetra-EGFP protein, which is tool large to enter the nucleus by diffusion.
We are currently elucidating the downstream effects of such nuclear translocation in host cells.