Nanosensing
Visualisation of subtle changes to cellular environments can be achieved using nanosensors
Nanosensors at a glance
Fluorescent nanosensors are spherical probes composed of an inert matrix with nanometre sized dimensions. They selectively respond to stimuli in their surroundings to transduce fluorescence signals to a detector and 'sense' environmental changes. Due to their small size, high signal-to-noise ratio and versatile inert matrix, fluorescent nanosensors can be thought of as powerful tools that represent an advance in optical sensor based technologies.
Applications of Nanosensing
Nanoparticles have been developed for sensing:
- pH
- Oxygen
- Temperature
- Proteins
- Water
And generating:
- Reactive Oxygen Species (ROS)
They have been developed with demonstrated application in a range of microenvironments and model organisms, which include mammalian cell lines (hMSCs & HeLa), nematodes (Caenorhabditis elegans, Pristionchus pacificus), Yeast (Saccharomyces cerevisiae), extracellular matrix (collagen) and microelectronics (microelectromechanical systems).
Figure 1. (A) Fluorescent Nanosensor principles and scanning electron microscopy (SEM) Images of (B) polyacrylamide and (C) silica sol-gel based nanosensors.
pH Sensitive Nanosensors
Our fluorescent pH-sensitive nanosensors are diagnostic probes synthesised from an inert polyacrylamide matrix and are capable of making accurate ratiometric measurements to:
- A high spatial (<50 nm) resolution
- A high temporal (<100 ms) resolution.
- A high pH resolution (± 0.17 pH units).
We have recently shown how extended dynamic range pH-sensitive nanosensors can be used to monitor real time rhythmic intestinal pH oscillations in the model organisms Caenorhabditis elegans (Figure 2) and P.pacificus (Figure 3) and determined the intracellular pH changes in yeast during glucose metabolism (Figure 2.)
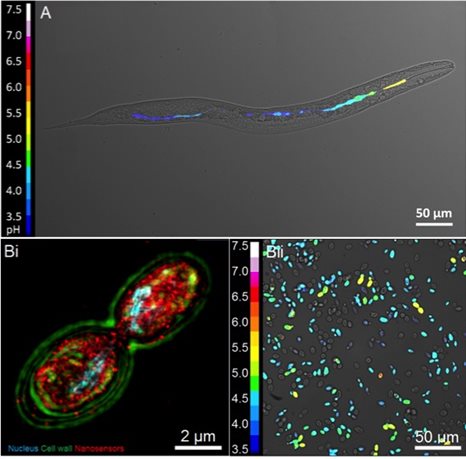
Fig 2. (A) Intestinal pH map of C. elegans, (Bi) subcellular delivery nanosensors to Saccharomyces cerevisiae and (Bii) corresponding pH distribution.
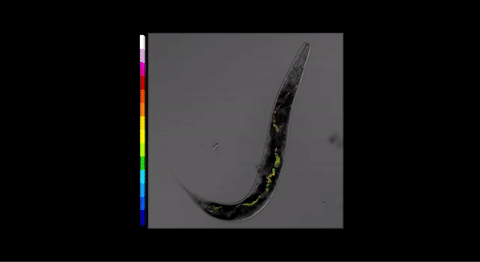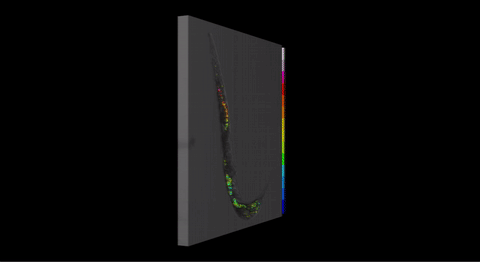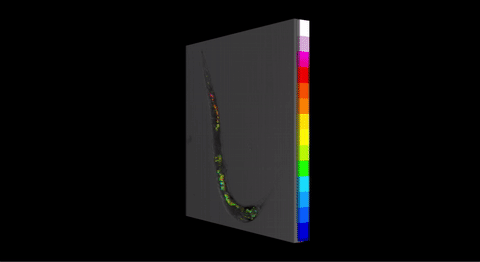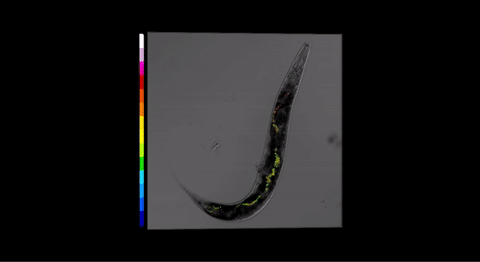
Fig 3. Three-dimensional pH map for P. pacificus
Oxygen Measurement
Oxygen sensitive nanosensors can be embedded in compressed collagen sheets, enabling innovative approaches for real-time resolution of oxygen gradients throughout 3D matrices useful for tissue regeneration.
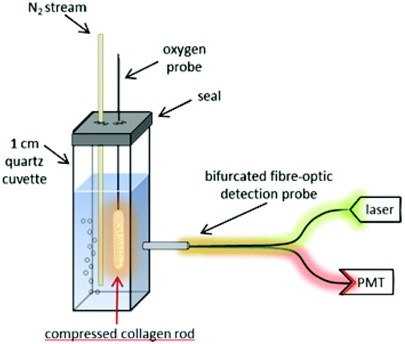
Figure 4. Methods for determining oxygen levels in compressed collagen using oxygen sensitive nanosensors.
Temperature Measurement
A custom designed microelectromechanical systems (MEMS) micro-hotplate, capable of operating at high temperatures (up to 700 °C), was used to thermo-optically characterise fluorescent rhodamine B (RhB) based silica sol-gel temperature-sensitive nanosensors (500 nm diameter).
The MEMS device used for this study could prove to be a reliable, low cost, low power and high temperature micro-hotplate for the thermo-optical characterisation of sub-micron sized particles from 0 – 145 °C, Figure 5.
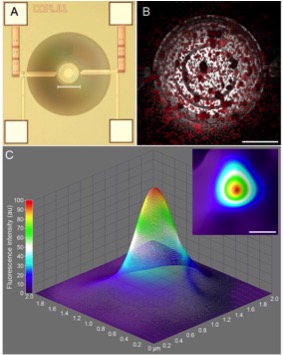
Figure 5. (A) Reflected light image of the silicon wafer of the MEMS device (scale bar = 150 μm), (B) temperature-sensitive nanosensors deposited on MEMS micro-hotplate heater (scale bar = 50 μm) and (C) Three and (inset) two dimensional surface plot intensity plots of nanosensors, imaged at 25 ̊C using confocal microscopy (scale bar = 500 nm)
Reactive Oxygen Species Generation
Fluorescent nanoparticles conjugated to zinc (II) or complexed porphyrins are capable of generating controlled amounts of ROS in human mesenchymal stem cells (hMSCs) upon irradiation with visible light. Control over ROS generation was demonstrated by: (1) attenuating the percentage of porphyrins on the nanoparticle surface and (2) modulating the number of light irradiation doses to the internalised nanoparticles. The degree of ROS production was visualised through use of a newly synthesised dye, which is chemically transformed into a fluorescent entity in the presence of ROS.
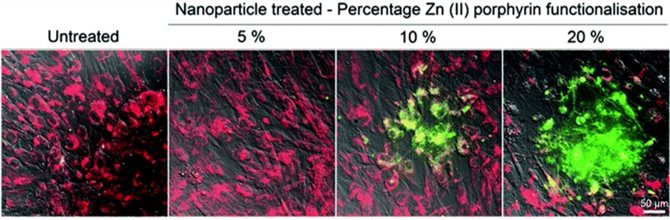 Figure 6. Fluorescence images for untreated hMSCs and hMSCs treated with 5, 10 and 20% Zn(II) functionalized nanoparticles, stained with BPTFMC and MitoTracker® red, irradiated with a single dose of light. BPTFMC, in the presence of H2O2, is converted to fluorescent green HTFMC. Scale bar = 50 μm.
Figure 6. Fluorescence images for untreated hMSCs and hMSCs treated with 5, 10 and 20% Zn(II) functionalized nanoparticles, stained with BPTFMC and MitoTracker® red, irradiated with a single dose of light. BPTFMC, in the presence of H2O2, is converted to fluorescent green HTFMC. Scale bar = 50 μm.
Publications of Interest
- Intracellular processing of silica-coated superparamagnetic iron nanoparticles in human mesenchymal stem cells, Harrison, R. P., Chauhan, V. M., Onion, D., Aylott, J. W. & Sottile, V., RSC Advances, 9, 3176–3184 (2019).
- Fluorescent nanosensors reveal dynamic pH gradients during biofilm formation, Hollmann, B., Perkins, M., Chauhan, V. M., Aylott, J. W. & Hardie, K. R., NPJ Biofilms Microbiomes, 7, 50 (2021).