Pharmacokinetics and Targeted Drug Disposition
We have significant collaboration between researchers involved with pharmacokinetics and drug disposition and those in nanomaterials formulation, nucleic acid therapeutics and RNA biology, allergy and infection.
Research in this theme includes Biopharmaceutics/Pharmacokinetics, Targeted Drug Delivery and Disposition, Drug Design and Development, and includes the following specific areas:
- Improvement of the efficiency, selectivity and safety profile of pharmacological therapy based on knowledge of pathophysiology of disease states and using advanced drug design, development and delivery methods.
- Targeted disposition achieved by specific formulation or route of administration approaches to increase the therapeutic benefits of potent drugs while minimizing the systemic toxicity.
- Intestinal lymphatic transport and targeting of drugs to lymph nodes to improve treatment outcomes of autoimmune diseases, cancer and HIV.
- Effects of disease states on pharmacokinetics and pharmacodynamics of drugs.
- Oral drug delivery, including the mechanisms of intestinal absorption and novel methods to overcome barriers to systemic bioavailability of orally administered drugs.
- Targeted delivery to lungs to improve treatment outcomes of a variety of disease of respiratory system
Projects in the theme have very direct translational significance and are accomplished by active links with clinical researchers at the hospital interface.
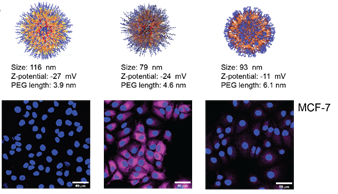
Functionalized Block Co‐Polymer Pro‐Drug Nanoparticles with Anti‐Cancer Efficacy in 3D Spheroids and in an Orthotopic Triple Negative Breast Cancer Model
Proposed publications:
-
Vincenzo Taresco, Thais F. Abelha Robert J. Cavanagh Catherine E. Vasey Akosua B. Anane‐Adjei Amanda K. Pearce Patrícia F. Monteiro Keith A. Spriggs Philip Clarke Alison Ritchie Stewart Martin Ruman Rahman Anna M. Grabowska Marianne B. Ashford Cameron Alexander Advanced Therapeutics 2020, https://doi.org/10.1002/adtp.202000103
- A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin. Lee JB, Radhi M, Cipolla E, Gandhi RD, Sarmad S, Zgair A, Kim TH, Feng W, Qin C, Adrower C, Ortori CA, Barrett DA, Kagan L, Fischer PM, de Moor CH, Gershkovich P. Sci Rep. 2019 Oct 31;9(1):15760. doi: 10.1038/s41598-019-52254-x
- Lipophilic activated ester prodrug approach for drug delivery to the intestinal lymphatic system. Lee JB, Zgair A, Malec J, Kim TH, Kim MG, Ali J, Qin C, Feng W, Chiang M, Gao X, Voronin G, Garces AE, Lau CL, Chan TH, Hume A, McIntosh TM, Soukarieh F, Al-Hayali M, Cipolla E, Collins HM, Heery DM, Shin BS, Yoo SD, Kagan L, Stocks MJ, Bradshaw TD, Fischer PM, Gershkovich P. J Control Release. 2018 Sep 28;286:10-19. doi: 10.1016/j.jconrel.2018.07.022
- Nieto-Orellana, A., H. Li, R. Rosiere, N. Wauthoz, H. Williams, C. J. Monteiro, C. Bosquillon, N. Childerhouse, G. Keegan, D. Coghlan, G. Mantovani and S. Stolnik. "Targeted PEG-poly(glutamic acid) complexes for inhalation protein delivery to the lung." Journal of Control Release 2019, 316: 250-262
- Linking in Vitro Lipolysis and Microsomal Metabolism for the Quantitative Prediction of Oral Bioavailability of BCS II Drugs Administered in Lipidic Formulations. Benito-Gallo P, Marlow M, Zann V, Scholes P, Gershkovich P. Mol Pharm. 2016 Oct 3;13(10):3526-3540. doi: 10.1021/acs.molpharmaceut.6b00597
- Vincenzo Taresco, Thais F. Abelha, Robert J. Cavanagh, Catherine E. Vasey, Akosua B. Anane‐Adjei, Amanda K. Pearce, Patrícia F. Monteiro, Keith A. Spriggs, Philip Clarke, Alison Ritchie, Stewart Martin, Ruman Rahman, Anna M. Grabowska, Marianne B. Ashford, Cameron Alexander Advanced Therapeutics 2020, https://doi.org/10.1002/adtp.202000103