Inspired by TG, we have created a library of second-generation semi-synthetic antivirals of which different representative molecules exhibit improved drug-like properties and safety whilst retaining antiviral efficacy against a range human and veterinary RNA viruses.
A major problem of all virus-targeting antivirals, such as oseltamivir, is the emergence of virus resistance which rapidly reduces their usefulness (Leung et al., 2024). Host-centric antivirals are much less susceptible to virus resistance since virus mutations cannot readily overcome its dependency on host cell functions. We previously detected no IAV resistance to TG after 10 serial cell passages (Goulding et al., 2020). Fig. 1 further demonstrated the distinct advantage of host-centric antivirals in not actively promoting virus resistance. We found no resistance of feline infectious peritonitis virus (FIPV), a feline coronavirus, to BO-65, a novel exemplar antiviral analogue, after 10 serial cell passages in sub-optimal antiviral concentration. In contrast, after 10 passages of FIPV in sub-optimal concentration of remdesivir, a nucleoside analogue used in clinical FIP, the resulting passage 10 FIPV were completely resistant to 1.0 μM remdesivir.
Our broad-spectrum antivirals could provide a key line of defence against future pandemics. IAV has been responsible for multiple pandemics in humans and panzootics in other species, as exemplified by the recent spillover of avian H5N1 influenza virus in wild and domestic birds to mammals (Peacock et al., 2024). We have identified compounds with potent activity against multiple influenza virus subtypes across different species, including bird flu virus (Fig. 2).

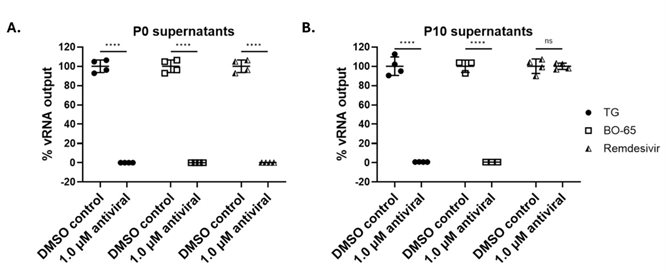
Fig. 1. Exemplar analogue BO-65 and TG at sub-optimal antiviral concentration did not induce FIPV resistance after 10 serial cell passages. CRFK cells were infected FIPV (strain DF2) at 0.2 MOI in infection medium (DMEM supplemented with 1 % P/S) for 2 h, washed with PBS and replaced with fresh medium (DMEM supplemented with 1 % P/S and 2 % FCS) containing sub-optimal concentrations of TG, BO-65 or remdesivir at 0.93 nM, 2.28 nM and 31.1 nM, respectively, for 24 h after which media from infected cells (P1) were collected, spun and used to infect the next round of freshly seeded CRFK cells. This process was repeated to generate P10 spun media. (A) CRFK cells were infected with stock FIPV (P0) at 0.2 MOI, washed with PBS after 2 h and incubated for 24 h in the presence of 1 µM TG, 1 µM T1, 1 µM remdesivir or DMSO control. (B) Likewise, CRFK cells were infected with equal volumes of P10 media containing virus for 2 h, washed with PBS and incubated for 24 h in the presence of 1 µM TG, 1 µM T1, 1 µM remdesivir or DMSO control. Viral RNA was extracted from 140 μl of spun media using the QIAamp Viral RNA Kit (Qiagen) and quantified by real-time, one-step RT-PCR (QuantiFast SYBR Green, Qiagen). Significance determined by two-way ANOVA, relative to the untreated control, on normalised data.
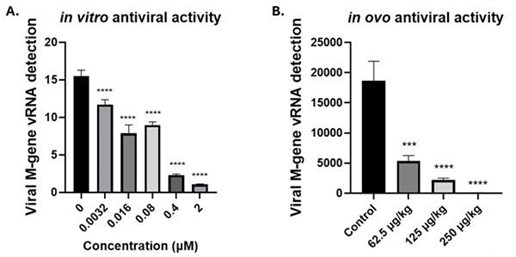
Fig. 2. Anti-avian influenza virus activity of semi-synthetic antivirals in vitro and in ovo. (A) DF-1 cells were infected with H6N1 virus at 1.0 MOI for 2 h before the infection medium (OptiMEM supplemented with 1 % P/S and 200 ng/mL TPCK trypsin) was replaced and the cell incubated in IM supplemented B125 at 2, 0.4, 0.08, 0.016 or 0.0032 uM for 24 h. (B) Ten day old dekalb white chicken eggs were simultaneously infected with a genetically modified highly pathogenic avian influenza virus (AIV09 with PR8 HA and NA) and treated with E173 at the indicated concentration (µg/kg), n=5/group. 10 mM stock suspended in DMSO was diluted in PBS and injected into the allantoic fluid. Allantoic fluid was collected 48 h post infection. (A, B) Viral RNA extracted from spun cell culture supernatant or the allantoic fluid (QIAamp Viral RNA minikit, QIAGEN) and virus copy number quantified by one step RT-qPCR (QuantiNova, QIAGEN). Significance determined by one-way ANOVA, relative to the untreated control.